Oh the irony!
Cutting CO2 emissions is…
And
Yes, that’s right, deadly man-made CO2 is the largest cooling agent of the stratosphere as demonstrated by this computer-modeled representation of stratospheric cooling rates:
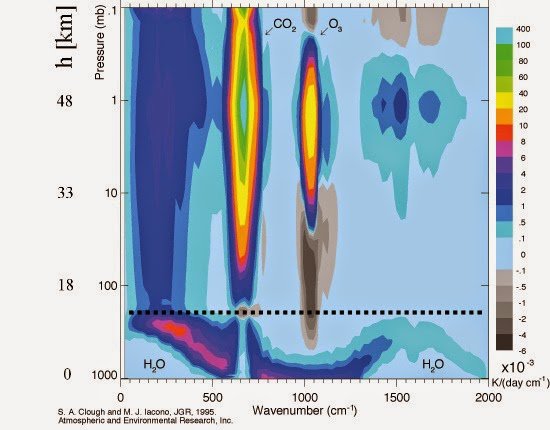
Image from blog article, originally in E M Smith’s article chiefio.wordpress.com/2014/06/01/le-chatelier-and-his-principle-vs-the-trouble-with-trenberth/
Below the Tropopause convection rules. Above it, radiative heat transport rules. Right off the bat, we have a big clue about why AGW (human caused ‘Global Warming’) is based on errors. The belief that radiative forcing at ground level ‘matters’ is simply shown a fantasy by the existence of the Troposphere. BY DEFINITION, it is convection, evaporation, condensation, clouds and rain that matter in the Troposphere. But lets look at that graph some more and pick up some interesting bits.
Only modelling, does show a null below the CO2, blocked channel.
I don’t buy the CFC argument, problem of lack of direct data where as usual there is lots of assertion but gaining hard data is somehow a step too hard. Looks to me more like the classic of PhD noticing something and getting the panics, we are all going to die. Seems rather likely ozone varies but we have no historic data. Dobson got the panics as I indicated last year, now’t like a pair of newspaper articles to show attitude change , here (updated post with most broken links repaired).
Also note the reported supposed recovery of Antarctica Ozone just happens to be as Antarctic ice is being awkward. What really causes what? I’m also mindful of radiation wavelength changing with temperature, cold Antarctica changes the rules.
Post by Tim






Been there, done that back in 2011 –

http://tinypic.com/r/15n0xuf/6
http://tinypic.com/r/zmghtu/6
The height of energy entry and exit from a fluid column in a gravity field has a critical role in setting the average temperature and temperature distribution within the column. A radiative atmosphere runs cooler, no if, no but, no maybe. In terms of cooling the atmosphere, warming near surface/cooling at altitude trumps warming near surface/cooling at surface every time. Without radiative gases, most of our atmosphere would boil into space. (Gas conduction is poor, without radiative cooling, the Tav of the bulk of the atmosphere would be set by surface Tmax)
Within our atmosphere, regarding net flux, radiative gases play double the role in cooling our atmosphere than they do in warming it. As our atmosphere exhibits strong vertical circulation, this fact alone means that AGW is a physical impossibility.
Most stratospheric cooling due to CO2? No news there, H2O ran out at the tropopause. Funny thing, strong vertical circulation also ceases at the tropopause. Gasp! Could radiative subsidence of air masses play a critical role in the speed of tropospheric circulation on the Hadley, Ferrel and Polar cells? If we increase radiative gases, the speed of tropospheric vertical circulation will increase, increasing the speed of surface cooling. Want your model to show increased surface warming? Simple. Hold the speed of vertical circulation constant for increasing radiative gas concentration…Total BS of course, but hey, Obamaclese is paying.
The state of ozone 10.09 in 2010 and in 1980.


http://exp-studies.tor.ec.gc.ca/cgi-bin/clf2/selectMap?lang=e&clf=2&printerversion=false&printfullpage=false&accessible=off&type1=de&day1=10&month1=09&year1=1980&howmany1=1&interval1=1&intervalunit1=d&hem1=g&type2=no&day2=10&month2=09&year2=2014&howmany2=1&interval2=1&intervalunit2=d&hem2=n&mapsize=100
Sorry 2014.
By the way, in September, will again be a record of ice in Antarctica.
http://arctic.atmos.uiuc.edu/cryosphere/antarctic.sea.ice.interactive.html
Let’s see current distribution of ozone in the northern hemisphere. You can see that the lock is on the Bay of Hudson.

It’s enough to snow was falling in the USA in September.
As of Thursday morning, up to 7 inches accumulated on portions of the Black Hills in South Dakota, and Cut Bank and Lewistown in Montana had their first snowfall of the season. The early snowfall has caused over 30,000 power outages in Calgary, Alberta, and threatens to cause power outages in the Midwest.
Back in the days when I was full of enthusiasm I discussed similar in my layman fashion:
http://www.globalwarmingskeptics.info/thread-249-post-10841.html#pid10841
People seem to only talk temperature these days, no science.
Nice to read this new stuff.
Ren is back! Thanks, a very interesting post!
@tchannon 😎 thanks for featuring the loudest bang in the otherwise sepulchral silence of climastrology 🙂
Just for the record: computer models invalidate games consoles. Gee!
Er, why would there be any people who “suddenly” get rid of mindcuffs, a mystery …
Us see also ozone during the solar minimum in 2009.

It is noteworthy that excess of ozone in the Southern magnetic pole. Such excess block the polar vortex.
With activists now arguing that the Montreal Protocol is the ‘successful’ model on which we should be basing our response to the ‘threat’ of climate change (http://www.theguardian.com/environment/georgemonbiot/2014/sep/11/stopping-climate-meltdown-needs-the-political-courage-that-saved-the-ozone-layer), it is timely to look back at 1985 when the Ozone Hole was discovered and examine the science which ‘proved’ that it was primarily due to stratospheric CFCs. The public were far less inclined to be sceptical of science in those days and far more inclined to believe the consensus opinion of scientists, believing it naturally to be based upon solid scientific investigation. But was it? Was the link between CFCs and ozone depletion – long term worldwide decline and ‘catastrophic’ Antarctic decline during SH Spring – really that well established? Roger Pielke has an interesting piece on this topic:
https://www.chinadialogue.net/article/show/single/en/5297
konrad, can you point at the regime change between vertical circulation cell and the other processes sitting above there?
The consideration of layers, either literal as in distinct slabs with specific identifying factors or notional as a way of breaking a continuum into computationally useful sections, as FEA or other lumped method. Or I think rather importantly as the dual of consensus climatic GCM, which everyone seems to indicate holds promise yet acts as a very hot potato.
More in the way of practical experiment must be possible, designing useful experiments is part of engineering and science. Doing this within resource is part of the hard trick.
For example, checking what happens under moderate vacuum although I suspect this becomes far beyond home experiment land, needs a lot of space and resource.
I expect most of the answers are already written down. Needle in haystack including what is trustable.
Question: why is there a distinct boundary between troposphere and stratosphere, the fundamental reasons?
Question: there is opinion the Antarctic plateau has no troposphere, how true is this?
Jamie says:”…back at 1985 when the Ozone Hole was discovered…”
====
“The ozone layer was discovered in 1913 by the French physicists…”
“Between 1928 and 1958 Dobson established a worldwide network of ozone monitoring stations, which continue to operate to this day. The “Dobson unit”, a convenient measure of the amount of ozone overhead, is named in his honor.”
The above from Wiki. The ozone hole was discovered by Dobson in the ’50’s. It was noticing the reduction in ozone that was found in ’85.
FWIW, the quote under the picture of the CO2 / other gasses modeled stratospheric cooling was used in the referenced posting (Hockeyschtick) but they referenced the source, my posting here:
No worries. Just a bit odd to read something that is oddly familiar 😉
Glad to see it getting some coverage.
The ‘net net’ is that the CO2 stratospheric ‘diamond’ is radiating a lot of heat away, while the ‘gap’ below the tropopause line shows CO2 doing no radiating in the troposphere. In short, CO2 does nothing to serface temps, but can cool the stratosphere some.
Troposphere is dominated by convection, precipitation, etc. Not IR / radiation. Water matters “down here”. CO2 cools “up there”.
Oops EMS. I must have missing the original in the feed from your blog.
I’ve added a link in the front page part..
@ E. M. Smith
Thanks for your great piece, I’ve been referencing this since you first published it, and tried to make others aware of it, as IMO it is one of the most cogent pieces ever written that the layman can understand.
Thank you tchannon for highlighting it again.
New:

• Blues: Mann2009 Temperatures with CO2 removed
• Oranges: Solar Cycle Deceleration accounts for half a degree Celsius
Suggested Exercise for Students: Replace CO2 with Sunspot Integral.
Here is the abstract from the paper that contained the figure at the top (emphasis added).
It is clear that the authors of the article conclude that their work supports overall warming of the atmosphere by CO2.
http://onlinelibrary.wiley.com/doi/10.1029/95JD01386/abstract
Uncle SAM’s Tip for leading students: CO2 being replaced by Sunspot Integral
Tim Folkerts says:
September 13, 2014 at 4:17 am
—————————————–
A line-by-line model cannot give the right answer as the speed of tropospheric convective circulation (and thereby energy transport away from the surface) must increase for increasing radiative gas concentrations.
You cannot just superimpose convection and evaporation on top of a static atmosphere radiative model.
“CO2 does what exactly?”
According to SAM, not much:

(new: global sea level pressure now included)
Its remarkable how papers which apparently contradict the ‘settled IPCC science’ have to insert the obligatory ‘get out of jail card’
“The radiative consequences of doubling carbon dioxide from the present level are consistent with these results. ”
Without this genuflection, the papers would not be published
“The principal effects of adding carbon dioxide are to reduce the role of the water vapor in the lower troposphere”
For a doubling of CO2 from present levels the ratio of H2O/CO2 goes from 2500 to 1250.
I would suggest that this change in the troposphere would have negligible thermal consequences.
Konrad likes testing over analysis but at 6:02am demonstrates not even performing a few minutes of research to see if radiosondes & satellite tests confirm line by line radiative transfer (LBLRTM) calculations in the real tropical and arctic atmospheres per Clough Iacano 1995 figure top post. Turns out they do “satisfactorily well”.
LBLRTM analysis residuals are on the order of measurement uncertainty from radiosonde and satellite in the tropical and arctic columns analyzed by Clough and Iacano 1995. Meaning observed results minus calculated results (the residuals) for the same atm. profile are small enough to prove the line by line radiative transfer method is accurate enough – at least for gov. work. Despite windy conditions.
Which of course supports 1938 Callendar was more correct over the test of time than Sir George Simpson about windy conditions – Konrad’s (and Stephen Wilde’s) speed of convective circulation debate nonetheless. The top post picture is reliable enough despite convective circulation conditions. Google string I used for many papers in this subfield: Clough Iacano 1995 residual. Plus there are more at ARM.gov
“This high sensitivity of the ATMOS instrument for the halocarbons makes it an interesting case for testing the simulations. Overall, we have found that LBLRTM reproduces very satisfactorily the spectral features of the ATMOS spectrum..”
While there are remaining issues (need more research paid for as usual), LBLRTM rigor of 1995 (picture in top post) has stood the test of time “very satisfactorily”.
Click to access coheur.pdf
Click to access ir_spectralradiance_tropicalpacific_jgr1997.pdf
Supporting and quantifying Tim Folkerts bolded 4:17am, research from the top post picture LBLRTM showing its results for +/- 20% water vapor, doubling of CO2 ppm and temperature increase in tropics (TRO) and subarctic (SAW) in Fig. 6 is here:
Click to access olr-modeling.pdf
What’s with the RHS y-axis in the first figure? Is it log[color]?
“Its remarkable how papers which apparently contradict the ‘settled IPCC science’ have to insert the obligatory ‘get out of jail card’ “
Or perhaps the paper DOES support “more CO2 = more warming”, and it is the secondary analysis by someone other than the authors who got it backwards.
Konrad says” A line-by-line model cannot give the right answer as the speed of tropospheric convective circulation (and thereby energy transport away from the surface) must increase for increasing radiative gas concentrations.”
As near as I can tell, the paper is not even addressing convection and makes no claims about it one way or the other. The graph shows how rapidly the atmosphere at a given altitude would cool due to specific frequencies of IR. It is not looking at other energy flows, like convection or warming due to absorption of visible light.
Tim
My point above is a more general one.
With the stress on “apparently contradict”
http://www.nature.com/nature/journal/v491/n7426/full/nature11579.html
http://hockeyschtick.blogspot.co.uk/2014/08/why-does-co2-cool-stratosphere-warm.html
We are supplied with contradicting information.
However the one thing that stays constant in the ‘IPCC science’ domain is that whatever is published it must not contradict the bottom line ‘CO2 is a dangerous pollutant ‘.
Its a bit like the Jesuits saying to Galileo;
Its alright to use mathematics that supposes the Earth circles the Sun as long as you make clear that in reality its the other way round
I’ll ask a stupid question. The atmosphere is not a constrained boiler that will explode if it gets to “hot”. If we add more gasses to the atmosphere, won’t the volume of the atmosphere just increase until it reaches equilibrium? And an increase in surface area of the atmosphere will allow for greater radiation, right?
The more radiative the atmosphere the more energy will be radiated to space from within that atmosphere.
Radiative loss from within the atmosphere represents a leakage of energy from the adiabatic exchange with the surface and so must WEAKEN convective overturning.
The more energy radiates to space from within an atmosphere the less is radiated to space from the surface because an increase exiting from within the atmosphere necessarily results in that much less being returned to the surface for radiation to space from the surface.
Jim S its not a stupid question.
However for each molecule of Carbon burned there is a combination with O2 to form CO2.
So the net result is the same number of atmospheric molecules, just less O2 and more CO2.
This is assuming that there are no other changes however there are a number of other competing processes going on,
It is time to help the IPCC by explaining why their models (CMIP etc) consistently fail to do a decent job of backcasting or pause in rising temeprature that has endured for at least 17 years.
The short answer is that the Arrhenius (1896) hypothesis concerning the effect of doubling [CO2] is false so any model that gives any weight to that hypothsis will perform poorly.
IMHO Chiefio is correct when he points out that while [CO2] affects temperature gradients in the stratosphere it can have no significant effect in the troposphere owing to the dominance of other heat transfer processes (phase change, convection an conduction). We don’t live in the stratosphere; we live in the lower troposphere which is opaque at wave number 667.
Lock in the south, which destroyed the wheat crop in southern Australia (temperature drop).

gallopingcamel says: September 14, 2014 at 1:27 am
“It is time to help the IPCC by explaining why their models (CMIP etc) consistently fail to do a decent job of backcasting or pause in rising temeprature that has endured for at least 17 years.
The short answer is that the Arrhenius (1896) hypothesis concerning the effect of doubling [CO2] is false so any model that gives any weight to that hypothsis will perform poorly.
IMHO Chiefio is correct when he points out that while [CO2] affects temperature gradients in the stratosphere it can have no significant effect in the troposphere owing to the dominance of other heat transfer processes (phase change, convection an conduction). We don’t live in the stratosphere; we live in the lower troposphere which is opaque at wave number 667.”
At STP the 15 microns CO2 (with H2O) optical depth is about 3 meters. It is never opaque if you have a big enough 15 micron laser! Besides with one of those, both CO2 and H20 would be elsewhere/elsewhen. Can you imagine the density of 2000 Kelvin gas molecules. The difference between 15 micron surface “radiance” up and down is zero, no flux. It is also zero with 250 ppmv CO2.
wildeco2014 says: September 13, 2014 at 10:48 pm
“The more radiative the atmosphere the more energy will be radiated to space from within that atmosphere. Radiative loss from within the atmosphere represents a leakage of energy from the adiabatic exchange with the surface and so must WEAKEN convective overturning. The more energy radiates to space from within an atmosphere the less is radiated to space from the surface because an increase exiting from within the atmosphere necessarily results in that much less being returned to the surface for radiation to space from the surface.”
What adiabatic exchange? For adiabat there is no exchange ever, adiabat is a reversable place keeper for energy. You seem to think that Earth/atmosphere energy/entropy must be conserved. WHY? It is a completely open system. It is the atmosphere only that discards unusable energy (entropy) to space. The surface is not involved, as it still has sensible heat to do interesting work (tornadoes with surface entropy)!
Will Janoschka waves caused by changing solar activity in the ionosphere and chemical changes in the stratosphere result of changes in solar radiation (galactic radiation simultaneously) govern the circulation in the atmosphere.
Need to compare the distribution of ozone with the magnetic field of the earth, in order to understand the importance of cosmic and solar radiation.


Please see drop in cosmic rays by 18% during the last magnetic storm. Conversely jump UVC and UVD.

@Will Janoschka
As someone who has been building and using lasers since 1970 I appreciate your comment about things never being truly “Opaque”. One usually thinks of concrete as opaque but the beam from the HIGS (High Intensity Gamma Source) can penetrate 20 feet of it.
The statement you objected to is better explained here:
@tchannon asked a question:
“Question: why is there a distinct boundary between troposphere and stratosphere, the fundamental reasons?”
Perhaps your question was for Konrad alone but here is my answer:
Our understanding of what determines the location of the tropopause has been been improved by Robinson & Catling. They have explained why the tropopause is defined by pressure (~0.1 bars).
The answer to your question is the title of their paper:
“Common 0.1 bar tropopause in thick atmospheres set by pressure-dependent infrared transparency”
Gallopingcamel

“What adiabatic exchange? For adiabat there is no exchange ever, adiabat is a reversable place keeper for energy. ”
Rising air cools and descending air warms. Both without addition or removal of energy. Instead, the form of energy changes from kinetic (heat) to gravitational potential energy (not heat) and back again.
The cause is gas density and weight variations in the horizontal plane which is in turn caused by uneven surface heating.
What so many miss is that the warming of air on the descent phase helps to reduce the rate of surface cooling and so raises the average surface temperature above that expected from the purely radiative S-B expectation. In the case of Earth by 33C.
That surface temperature rise is a consequence of mechanical adiabatic overturning and not downward IR.
Some say that because the process nets out to zero there is no effect on surface temperature but that is wrong for a scenario where there is a constant flow of new (solar) energy through the system.
What happens is that because solar energy arrives constantly the first ascent of the very first convective cycle fails to cool the surface. Instead it reduces the energy lost to space by converting it to gravitational potential energy during uplift. GPE does not radiate.
Then, once one completes the first descent of the very first convective cycle the surface continues to receive ongoing insolation but in addition it is receiving energy from that descending air so the surface temperature must then rise.
That additional surface energy is not availablre for radiation to space because it is immediately taken up in the next convective ascent.
Meanwhile, new insolation continues at the same rate as before so the surface temperature cannot drop back again.
The system equilibriates at a given strength of convective overturning and NOT at a given level of radiative flux though an increased radiative flux within the atmosphere is indeed an inevitable consequence of the mechanically enhanced surface temperature.
GHGs then cause more radiation to space from within the atmosphere but that reduces the energy returning to the surface on descent so convective overturning is weakened by exactly as much as the GHGs radiate to space.
The reduction in energy returning to the surface reduces radiation from the surface to space by exactly as much as the increase in radiation ftrom the atmosphere to space.
It is a self cancelling process at the expense of a miniscule circulation change.
gallopingcamel says, September 14, 2014 at 2:49 pm:
“Our understanding of what determines the location of the tropopause has been been improved by Robinson & Catling. They have explained why the tropopause is defined by pressure (~0.1 bars).
The answer to your question is the title of their paper:
“Common 0.1 bar tropopause in thick atmospheres set by pressure-dependent infrared transparency””
The energy that Earth has to get rid of to keep up with the input needs to be brought up (by convection) to a level from where it can be radiated directly to space. Some of the energy is radiated out straight from the solid/liquid surface, some from the surface air layer and some from every layer of the troposphere going up to the tropopause, the top of convective transfer.
What Robinson & Catling are hypothesizing is simply that at the point in an atmospheric air column where the pressure becomes low enough (100 mb) so that ‘the rest’ of this mass-transferred energy can be finally radiated out directly to space, then convection has done its job and can reach no higher (its upward momentum has decelerated to zero, no more ‘surplus’ energy) – setting the effective tropopause level.
Their hypothesis about IR optical depth and air pressure is only about determining tropopause height, the boundary between the convective regime of the lower atmosphere and the radiative regime of the upper atmosphere. The troposphere is ALL about convection moving the energy. That’s their point. The air pressure – according to them – simply decides how high up convection needs to bring the energy.
There is, however, a pretty obvious weakness to their 0.1 bar argument. Earth’s tropopause is ONLY at around 0.1 bar in the tropics (16-17 km) and nowhere else. (And even here it varies a bit with surface heating and convective/evaporative response.) In the middle and high latitudes the tropopause is rather at around 0.3 bar (even much lower around the poles in winter), the global average being in the vicinity of 0.2. You see this also quite clearly in their Figure 1. The global tropospheric lapse rate slope ends at 0.2 bar, not at 0.1. That’s a 4 km difference in altitude. On average. In reality, the tropical tropopause is nearly twice as lofty as the high latitude one. This difference is all about surface heating/evaporation.
Stephen 4:14pm – Your assertions are unsupported by natural observations and meteorological analysis. The top post picture is built from purely radiative line by line radiative transfer rigorous analysis from 1995. Many papers cite the work, it has stood the test of time.
That same picture can be produced including your natural convective “mechanical overturning” effects with either radiosonde or satellite measurements “satisfactorily well” in both tropical and subarctic regions. There is negligible effect on surface T – meaning within instrumental error – then from your convective “mechanical overturning” and certainly not a 33K result from such effect.
Conclusion: Stephen’s statement “what so many miss” is incorrect. Top post picture confirms radiative physics alone can explain the measured 33K to within instrument error and confidence intervals. Any global convective “mechanical overturning” effect on surface temperature is less than instrumental error which is the reason it is referred to as ~adiabatic. Whatever global convection goes up, comes down meaning global Tmean remains unchanged in the surface energy balance by any effects of convective “mechanical overturning”.
Since the radiative line by line analysis is proven acceptable by physical test, it can be used to better understand natural effects by analytically varying water vapor percentage and radiative active species ppm in the atmosphere. That is shown for example by Fig. 6 in the last paper I cited at 2:54pm.
You can see a very similar distribution of ozone in the northern hemisphere as last year, the anomalies are only a stronger.
http://exp-studies.tor.ec.gc.ca/cgi-bin/clf2/selectMap?lang=e&clf=2&printerversion=false&printfullpage=false&accessible=off&type1=de&day1=12&month1=09&year1=2013&howmany1=1&interval1=1&intervalunit1=d&hem1=n&type2=de&day2=12&month2=09&year2=2014&howmany2=1&interval2=1&intervalunit2=d&hem2=n&mapsize=100
okulaer, September 14, 2014 at 4:32 pm
Sorry, okulaer is Kristian.
Trick says, September 14, 2014 at 4:54 pm:
“Whatever global convection goes up, comes down meaning global Tmean remains unchanged in the surface energy balance by any effects of convective “mechanical overturning”.”
Global Tmean is not set by convective/evaporative cooling, Trick. It is set by the opposition to convective/evaporative cooling by the weight of the atmosphere on top of the surface. The radiatively active gases in the atmosphere aids convective cooling of the surface by working towards warming low (absorption from surface) and cooling high (emission to space).
This is the simple reason why Earth’s global surface is on average 90K warmer than the Moon’s. In spite of the net radiative cooling effect produced by the IR-active gases in the atmosphere.
Try this
New paper finds water vapor in the troposphere controlled by natural processes, not CO2
http://hockeyschtick.blogspot.co.uk/2014/09/new-paper-finds-water-vapor-in.html
Kristian 5:47pm: “Global Tmean is not set by convective/evaporative cooling, Trick.”
Concur. As shown by the top post figure and 1st law analysis.
“The radiatively active gases in the atmosphere aids convective cooling of the surface by working towards warming low (absorption from surface) and cooling high (emission to space).”
Yes, as shown by 1st law balance in basic atmosphere radiation text books and confirmed in the top post 1995 rigorous analysis LBL. Addition of IR active gas ppm has the effect shown in Fig. 6 of the paper I posted 2:45pm calculated with the same LBLRTM supported by test results as the top post figure.
Interpret the red,yellow area labeled CO2 confirming the cooling effect on the stratosphere by near surface IR active gas in the lines as shown. In effect providing shade in that area from terrestrial surface emissions for the global stratosphere in these lines. This is why so much interest in providing a better field of temperature measurements in the stratosphere to better determine its long term T trends. This is the effect keeps Earth global surface Tmean measured approx. 33K higher T than with an extremely optically thin atm.
To get to the moon’s global Tmean of approx. 197K from Diviner understanding, the work gallopingcamel is doing pushes it forward – other thread. Airless surface powder optical depth is a factor there.
Trick says, September 14, 2014 at 8:58 pm:
“This is the effect keeps Earth global surface Tmean measured approx. 33K higher T than with an extremely optically thin atm.”
No.
Again: “[Surface temperature] is set by the opposition to convective/evaporative cooling by the weight of the atmosphere on top of the surface. The radiatively active gases in the atmosphere [aid] convective cooling of the surface by working towards warming low (absorption from surface) and cooling high (emission to space).
This is the simple reason why Earth’s global surface is on average 90K warmer than the Moon’s. In spite of the net radiative cooling effect produced by the IR-active gases in the atmosphere.”
The IR-active gases ALSO cools in the troposphere. That’s what they do. They absorb IR, yes, but don’t get to turn the absorbing tropospheric air layer any warmer in doing so, because convection will automatically and instantly bring the energy up through mass transfer, always maintaining the temperature profile – the energy will thus simply end up at the level from where it can finally be radiated to space. The emitting ability of the IR-active gases, however, does help cool the troposphere and hence the surface by ridding the system of the energy altogether.
In other words, if the troposphere didn’t absorb IR, that doesn’t mean it wouldn’t warm. It would still warm convectively. But if the troposphere didn’t emit IR to space, then that does mean it couldn’t adequately cool.
Kristian says: “In other words, if the troposphere didn’t absorb IR, that doesn’t mean it wouldn’t warm. It would still warm convectively. But if the troposphere didn’t emit IR to space, then that does mean it couldn’t adequately cool.”
The atmosphere must either
* both emit and absorb IR
* Neither emit nor absorb IR
Just one would violate Kirchhoff’s Radiation Law & the 2nd Law of thermodynamics.
If the atmosphere does neither, then it could warm by conduction/convection up to the surface temperature. But the surface temperature would be only average ~ 255-270 K (depending on what assumptions you make about the albedo & emissivity). The surface simply radiates unimpeded straight to space. So the atmosphere would only warm to this rather chilly level
If the atmosphere does both, then the cool top of the atmosphere radiates poorly to space and the surface must be warmer to radiate more effectively to space. This leads to the observed and predicted ~ 288 K average temperature.
Tim is employing the classic ‘goldfish memory’ tactic once again, I see. Very much favoured by warmists without any real arguments. Round and round we go. Tens of times he’s been told how he can’t just remove convective loss and then go straight to a purely radiating surface as long as there is an atmosphere in place, resting on top of the solar-heated surface. That the world doesn’t work like a mathematical equation. That the surface will have to become progressively warmer (by accumulating incoming energy) so as to be able to maintain the upward temp gradient and thus an adequate convective loss in trying not to overheat. Suppress convection from a heated surface surrounded by a fluid in a gravity field and you get warming, not cooling. But of course, every single time he ‘forgets’ about this and starts all over with his silly talking points as if seeing the world for the first time each and every lap around his fishbowl.
IR-active gases in the atmosphere help cool the Earth system, Tim. There is no question. They help keep it stable. Without them, there could either not be an atmosphere at all, or the system as a whole could never reach a steady state (a dynamic equilibrium).
H2O first and foremost, in all its forms, reflects and absorbs a large portion of the incoming solar heat flux, preventing it from ever reaching the surface. Both H2O and CO2 aid in convective cooling of the surface by net absorption low and net emission high. And both H2O and CO2 let the atmosphere, being warmed convectively from the surface and by radiative absorption from the Sun, cool to space by radiation.
This is all so basic and obvious. You’ll see it too. If you only dare to take it in.
The MASS of the atmosphere alone is what makes Earth’s global surface so much warmer on average than the Moon’s. In spite of the cooling radiative contributions of the IR-active gases.
Descending air warms adiabatically (without addition of new energy). That means the warming is not attributable to absorption of solar radiation, absorption of IR from the ground or conduction from the ground.
In that situation the only way that warming can occur is by changing the form of the energy carried by the air from gravitational potential energy (which is not heat and does not radiate) to kinetic energy (which does manifest as heat and does radiate).
The convective uplift of air does not cool the surface below the S-B figure because new energy continues to arrive to replace it.
Instead, the energy required by uplift is taken from the energy that would otherwise radiate to space and so that energy remains within the system to warm the air on the subsequent descent and thereby slow the cooling of the surface which must then rise 33C above S-B.
Until the implications of that are appreciated one cannot grasp the reality which I described above.
Kristian 9:38pm: “[Surface temperature] is set by the opposition to convective/evaporative cooling by the weight of the atmosphere on top of the surface.”
Nonsense Kristian, you were right the first time at 5:47pm: “Global Tmean is not set by convective/evaporative cooling…” This is confirmed in the top post chart analysis from 1995 and basic application of 1st law. To disagree with that rigorous analysis, you need to defeat the first law, LBLRTM and optical depth testing. Tough order, they all have 19 more years of confirmatory atmosphere testing and data analysis.
“This is the simple reason why Earth’s global surface is on average 90K warmer than the Moon’s”
Not observed reason. The moon global Tmean is observed around 197K by Diviner. An extremely optically thin atmosphere on earth by 1st law and the LBLRTM analysis in top post would have a global surface Tmean around 58K above the moon (around 255K as observed by satellite) because Earth surface would have been protected and not been pounded into as much powder as observed on moon. Negligible diffraction on earth and not negligible diffraction on the moon.
Add the IR active ingredients to increase optical depth up to today’s optically thick Earth atm. and find Earth global Tmean 288K by 1st law & LBLRTM as used in the top post analysis which agrees with thermometer field & the top post picture.
12:14am: “Tens of times he’s been told how he can’t just remove convective loss and then go straight to a purely radiating surface as long as there is an atmosphere in place, resting on top of the solar-heated surface. “
Tens of times incorrectly as the top post confirms. The top post LBLRTM analysis confirms CAN remove convection. Convection is confirmed adiabatic by that picture.
“Both H2O and CO2 aid in convective cooling of the surface..”
Again, the top post LBLRTM analysis confirms there is no global convective cooling of the surface or it wouldn’t match the radiosondes and satellites data to within instrumental accuracy.
“The MASS of the atmosphere alone is what makes Earth’s global surface so much warmer…”
Yes as optical depth increases. Because all solid, liquid, gas mass at all frequencies, at all temperatures, at all times emits radiation though in varying amounts given by the Planck distribution which is always nonzero. This is why LBLRTM ultimately works so well according to observations.
Stephen 12:26am “Descending air warms adiabatically (without addition of new energy). That means the warming is not attributable to absorption of solar radiation, absorption of IR from the ground or conduction from the ground.”
Concur. The top post picture confirms this by LBLRTM analysis. You will find this in the texts you haven’t as yet cracked open.
“kinetic energy (which does manifest as heat and does radiate).”
Kinetic energy does not radiate. Text book study will prevent you making this mistake. Mass radiates; amount by Planck distribution. Nothing manifests itself by heat which is nonexistent in nature. No manifestation of heat (in joules) anywhere by anything at any time in nature.
“…thereby slow the cooling of the surface which must then rise 33C above S-B.”
Slowing the cooling of the surface is not possible by adiabatic convection as confirmed by top post picture analysis agreeing with atmosphere measurements. The surface CANNOT ever be above S-B as 100+ years of confirmatory testing says temperature is equal S-B prediction; IR thermometers brightness temperature always confirmed by thermometers.
Stephen Wilde says, September 15, 2014 at 12:26 am:
“Descending air warms adiabatically (without addition of new energy). That means the warming is not attributable to absorption of solar radiation, absorption of IR from the ground or conduction from the ground.
In that situation the only way that warming can occur is by changing the form of the energy carried by the air (…)”
Once again, no, Stephen. Descending air warms adiabatically WITH an addition of new energy, only not HEAT. That’s what ‘adiabatic’ means. For the hundredth time. The energy it delivered to the surrounding atmosphere on ascent it gets back on descent. The energy is transferred across the adiabatic barrier in the form of WORK – expansion going up, compression coming down.
“(…) from gravitational potential energy (which is not heat and does not radiate) to kinetic energy (which does manifest as heat and does radiate).”
HEAT is not something that is contained WITHIN a system, Stephen. You mean ‘temperature’. Kinetic energy manifests as temperature. Potential energy does not. Heat is a TRANSFER of energy by virtue of a temperature difference.
“(…) the energy required by uplift is taken from the energy that would otherwise radiate to space and so that energy remains within the system to warm the air on the subsequent descent and thereby slow the cooling of the surface which must then rise 33C above S-B.”
No, Stephen. The energy brought up into the troposphere from the surface is radiated to space. It escapes the system. It does not contribute to the warming of descending air. The adiabatic cycle is a zero-sum game. It is not what warms or cools the atmosphere. That’s diabatic heating and cooling. What forces the surface to warm beyond pure solar radiative equilibrium is the mass of the atmosphere opposing convective/evaporative cooling. Not air warming adiabatically on descent.
Kristian says: “That the surface will have to become progressively warmer (by accumulating incoming energy) so as to be able to maintain the upward temp gradient … ”
If the atmosphere is transparent to IR, then the surface will have to become progressively warmer so as to me able to radiate as much IR to space as it absorbs from the sun. That is the most basic requirement — dictated by conservation of energy.
As seen in another recent post about the moon (https://tallbloke.wordpress.com/2014/08/27/extending-a-new-lunar-thermal-model-part-ii-modelling-an-airless-earth/), the average surface temperature to get radiative balance is seen to be well below 288 K — confirmed both experimentally and by the calculations in that post.
Adding a transparent atmosphere cannot change that radiative balance in the slightest, and hence cannot raise the average surface temperature (other than a slight rise due to the Holder inequality as the temperature gets a little cooler on the day side and a little warmer on the night side).
There is a FUNDAMENTAL requirement to conserve energy. There is NO fundamental requirement to create a specific temperature gradient. Thus conservation of energy (and a 255-270 K average temperature) is the ultimate factor for determining the global average temperature, not the adiabatic lapse rate. (And since such a hypothetical atmosphere does not exist anywhere that we can study it, there is no reason to spend too much time trying to imagine what sort of temperature profile might exist there).
” … so as to be able to maintain … an adequate convective loss in trying not to overheat.
There will be NO (net) convective loss on such a planet with a transparent atmosphere! The net lose from the atmosphere to space is zero. The net input to the atmosphere from the sun is zero. Hence the net input to the atmosphere from the ground must ALSO be zero. There would be some convective transfer from surface to atmosphere during the day, and some convective transfer back from atmosphere to surface during the night. But the net result is zero.
The surface doesn’t “overheat” because it can shed energy by radiating IR straight to space, limiting the average temperature to ~ 255-270 K.
Trick says: September 15, 2014 at 12:39 am
(Kristian 9:38pm: “[Surface temperature] is set by the opposition to convective/evaporative cooling by the weight of the atmosphere on top of the surface.”)
“Nonsense Kristian, you were right the first time at 5:47pm: “Global Tmean is not set by convective/evaporative cooling…” This is confirmed in the top post chart analysis from 1995 and basic application of 1st law. To disagree with that rigorous analysis, you need to defeat the first law, LBLRTM and optical depth testing. Tough order, they all have 19 more years of confirmatory atmosphere testing and data analysis.)
This is Trick’s fantasy.
In an open system such as Earth and its atmosphere any application of 1st law is false. Thas been no rigorous analysis. The, Line By Line Radiative Transfer Model is but a “deliberate” corruption of all the carefull measurements contained in the USAF HiTran data base. Optical depth is a measure only of the attenuation of rapid variations of thermal radiative flux, never the attenuation of radiative flux itself of a material at or near thermodynamic equilibrium. Such would be a direct violation of Kerchhoff’s Laws of Thermal Raduation.
(“This is the simple reason why Earth’s global surface is on average 90K warmer than the Moon’s”)
“Not observed reason.”
Reason (conjecture) is never part of observation, only an excuse (mostly incorrect) for the observation!
“The moon global Tmean is observed around 197K by Diviner. An extremely optically thin atmosphere on earth by 1st law and the LBLRTM analysis in top post would have a global surface Tmean around 58K above the moon (around 255K as observed by satellite) because Earth surface would have been protected and not been pounded into as much powder as observed on moon. Negligible diffraction on earth and not negligible diffraction on the moon.”
1st law and the LBLRTM do not apply to open systems. Can you give any possible definition to your use of the word “diffraction” on the moon?
“Add the IR active ingredients to increase optical depth up to today’s optically thick Earth atm.”
Only Trick would claim that adding dispersive matter to a gas would increase optical depth. All others would agree that such addition must decrease optical depth.
“find Earth global Tmean 288K by 1st law & LBLRTM as used in the top post analysis which agrees with thermometer field & the top post picture.”
1st law and the LBLRTM do not apply to open systems. The top post and its reference paper both disagree with your nonsense.
(12:14am: “Tens of times he’s been told how he can’t just remove convective loss and then go straight to a purely radiating surface as long as there is an atmosphere in place, resting on top of the solar-heated surface. “)
“Tens of times incorrectly as the top post confirms.”
The top post claims no such thing!
“The top post LBLRTM analysis confirms CAN remove convection. Convection is confirmed adiabatic by that picture”
Correct LBLRTM analysis must only confirm convection! There is no other way for WV to become part of the atmosphere.
(“Both H2O and CO2 aid in convective cooling of the surface..”)
“Again, the top post LBLRTM analysis confirms there is no global convective cooling of the surface or it wouldn’t match the radiosondes and satellites data to within instrumental accuracy.”
There is no top post LBLRTM analysis.
(“The MASS of the atmosphere alone is what makes Earth’s global surface so much warmer…”)
“Yes as optical depth increases. Because all solid, liquid, gas mass at all frequencies, at all temperatures, at all times emits radiation though in varying amounts given by the Planck distribution which is always nonzero. This is why LBLRTM ultimately works so well according to observations.”
What total nonsense. Gravitational attraction alone compresses the N2 and O2 in the lower atmosphere resulting in an increase of lower temperature. This is a thermostatic pressure and temperature gradient both increasing at lower atmosphere No thermdynamic energy transfer is involved.
okulaer, September 14, 2014 at 4:32 pm
“Earth’s tropopause is ONLY at around 0.1 bar in the tropics (16-17 km) and nowhere else.”
The trouble with one dimensional models of the atmosphere is that they are based on global averages just like Trenberth (2009):

You complain that the R&C model does not explain the variation of the height of the tropopause with latitude. You are right but such models don’t explain diurnal or seasonal variations either. Perhaps that was the point of the “ren” comment above.
R&C say that radiation is the dominant energy transfer process in the stratosphere which accounts for the positive stratospheric lapse rate on six of the seven bodies in the solar system that have significant atmospheres. They have a plausible explanation for the anomalous stratospheric lapse rate on Venus.
I was intrigued by the N&K Unified Theory of Climate that said pressure is the primary variable determining the surface temperature of bodies within our solar system. N&K produced equations that were accurate at the surface rocky planets but were not so good at predicting temperatures at arbitrary altitudes. I was looking for something better and Robinson & Catling delivered it:
I see the R&C model as an improvement on the N&K equations. Eventually, other workers will improve on the R&C model. That is how science works. Right now I am trying to reproduce the R&C model using Finite Element Analysis software. It may be possible to add cloud layers.
You point out the deficiencies of the R&C model but is a better model is available? Are you interested in working with me to improve the R&C model?
I don’t agree with all of what Trick said, but Will’s rebuttal is FULL or problems!
“In an open system such as Earth and its atmosphere any application of 1st law is false. ”
No, there are plenty of ways to apply the first law (ie conservation of energy) in such situations. The only real challenge comes when mass can flow in/out from the system, but this doesn’t happen for the system consisting of the earth & its atmosphere. Then the standard 1st Law is exactly what is needed
ΔU = Q-W
This also applies to any given “parcel” of air as well.
“Optical depth is a measure only of the attenuation of rapid variations of thermal radiative flux, never the attenuation of radiative flux itself of a material at or near thermodynamic equilibrium.”
Optical depth is a measure of how quickly light of a given frequency drops off as you go through a material. For example, red plastic has a short optical depth for blue light, but a long optical depth for red light. The definition has nothing to do with how rapid any variations might be.
As another example, if you shoot a CO2 laser through the atmosphere, the beam will be attenuated by the CO2 in the air.
“Such would be a direct violation of Kerchhoff’s Laws of Thermal Raduation.”
No, This law state that for a material at thermal equilibrium, the absorptivity of EMR at a given wavelength = the emissivity at that wavelength. In other words, you don’t need one emissivity to calculate absorption and another to calculate emission.
Now it is true that within a system at thermal equilibrium, there is no net transfer of thermal EMR anywhere. But this is not really what K’s Law is getting at. It is a much more powerful statement, that if an object is good at emitting EMR to an object that is cooler, then it is equally good at absorbing EMR from an object that is warmer. If this were not true, THEN we would have a violation of K’s Law.
“Only Trick would claim that adding dispersive matter to a gas would increase optical depth. All others would agree that such addition must decrease optical depth.”
No, only someone who knows the definition of “optical depth” would make such a claim. Read up on hte topic here: http://en.wikipedia.org/wiki/Optical_depth
In particular, a transparent atmosphere has zero optical depth. A gas sample that attenuates 1/e of a beam has a depth of 1. A gas sample that attenuates 1/e^2 of a beam has a depth of 2. The greater the attenuation, the greater that optical depth.
Trick says, September 15, 2014 at 12:39 am:
“Nonsense Kristian, you were right the first time at 5:47pm: “Global Tmean is not set by convective/evaporative cooling…””
These two statements are two sides of the same coin. The one follows the other. In succession. You only have to read what I wrote to see it: “Global Tmean is not set by convective/evaporative cooling, Trick. It is set by the opposition to convective/evaporative cooling by the weight of the atmosphere on top of the surface.” This simple insight draws from relatively basic physical principles, Trick. Gas dynamics.
The radiative properties of IR-active gases in the atmosphere do not oppose or impede convective cooling of the surface, Trick. Quite he opposite. The mass of the atmosphere, however, clearly does. By being able to warm (‘heat capacity’). And by exerting pressure on the surface (weight).
You can deny this until you’re blue in the face, Trick. It is still true.
gallopingcamel says, September 15, 2014 at 5:48 am:
I do not dismiss R&C’s hypothesis. I’m merely pointing out that their 0.1 bar relationship seems to not take into account the tight coupling between surface heating/evaporation and upward buoyant momentum as a cause for tropopause height. On Earth, their 0.1 bar idea only seems to work in the tropics. It doesn’t work for the global mean. The global mean tropopause height is rather at 0.2 bar.
Why does it (seem to) work in the tropics? Coincidence? I don’t know. Maybe there’s something to what they say, that IR optical depth does have a bearing on ‘final’ tropopause height. At least it might constitute one part of the answer. It does seem plausible that optical depth influencing the escape of IR to space from a rising parcel of air does contribute to setting some kind of limit to how high buoyant momentum can carry that parcel.
On Earth, however, I still favour the surface heating > convective/evaporative response explanation as the main one for tropopause height:
Tim Folkerts says: September 15, 2014 at 6:14 am
“I don’t agree with all of what Trick said, but Will’s rebuttal is FULL or problems!”
(“In an open system such as Earth and its atmosphere any application of 1st law is false. ”)
“No, there are plenty of ways to apply the first law (ie conservation of energy) in such situations. The only real challenge comes when mass can flow in/out from the system, but this doesn’t happen for the system consisting of the earth & its atmosphere. Then the standard 1st Law is exactly what is needed. ΔU = Q-W This also applies to any given “parcel” of air as well.”
In the Earth’s troposphere we have the the lowest temperatures around. All sensible heat is already entropy at that temperature all latent heat becomes entropy upon condensation. All this entropy to be dispatched to space via atmospheric EMR. None returns to the surface at a higher temperature by any means whatsoever.. In an open system such as Earth and its atmosphere any application of 1st law is false.
(“Optical depth is a measure only of the attenuation of rapid variations of thermal radiative flux, never the attenuation of radiative flux itself of a material at or near thermodynamic equilibrium.”)
“Optical depth is a measure of how quickly light of a given frequency drops off as you go through a material. For example, red plastic has a short optical depth for blue light, but a long optical depth for red light. The definition has nothing to do with how rapid any variations might be.”
The red plastic absorbs blue light “only” if not at radiative (thermodynamic) equilibrium of that blue light. How does that apply in this atmosphere?
“As another example, if you shoot a CO2 laser through the atmosphere, the beam will be attenuated by the CO2 in the air.”
Atmospheric CO2 does not absorb at the wavelength of a CO2 laser, beam or not!
“Such would be a direct violation of Kerchhoff’s Laws of Thermal Raduation.”
No, This law state that for a material at thermal equilibrium, the absorptivity of EMR at a given wavelength = the emissivity at that wavelength. In other words, you don’t need one emissivity to calculate absorption and another to calculate emission.”
That is but one of Kerchhoff’s Laws of Thermal radiation regarding identical antenna gain “in and out” at each frequency and in each directionl Go read the others!
“Now it is true that within a system at thermal equilibrium, there is no net transfer of thermal EMR anywhere. But this is not really what K’s Law is getting at. It is a much more powerful statement, that if an object is good at emitting EMR to an object that is cooler, then it is equally good at absorbing EMR from an object that is warmer. If this were not true, THEN we would have a violation of K’s Law.”
Not thermal, but thermodynamic and radiative equilibrium where any constant energy transfer from anything to anything else is accepted for a stable and spontaneous equilibrium.
Any matter at radiative (thermodynamic) equilibrium even opaque must be radiating the same amount of flux to lower temperature as it absorbs from a higher temperature, else energy must transfer to or from that mass violating equilibrium. Tim your Kenetic theory of everything cannot change definitions.
(“Only Trick would claim that adding dispersive matter to a gas would increase optical depth. All others would agree that such addition must decrease optical depth.”)
“No, only someone who knows the definition of “optical depth” would make such a claim. Read up on hte topic here: http://en.wikipedia.org/wiki/Optical_depth”
The opital depth of any matter at any wavelength is the actual measured distance where nonequlibriun attenuation is (1- 1/e)! This then becomes an dimensionless “one” OD, so that exponentials and logarithms may be used. This can never be used anywhere in this atmosphere because there is no altitude where one OD is a constant for any frequency..
“In particular, a transparent atmosphere has zero optical depth. A gas sample that attenuates 1/e of a beam has a depth of 1. A gas sample that attenuates 1/e^2 of a beam has a depth of 2. The greater the attenuation, the greater that optical depth.”
You fogot a (to) preceeding the (1/e) or (1/e^2). attenuation. A Pascalian vacuum has infinite optical depth at all frequencies. It aways has zero optical depths. at every length. Notice the trailing (s). Tim why must you get everything backwards?
Kristian said:
“Once again, no, Stephen. Descending air warms adiabatically WITH an addition of new energy, only not HEAT. That’s what ‘adiabatic’ means. For the hundredth time. The energy it delivered to the surrounding atmosphere on ascent it gets back on descent. The energy is transferred across the adiabatic barrier in the form of WORK – expansion going up, compression coming down.”
Kristian, most of what you say is correct but that bit is not and it skews the rest of your interpretation of reality.
“An adiabatic process is a process that occurs without the transfer of heat or matter between a system and its surroundings.”
http://en.wikipedia.org/wiki/Adiabatic_process
Thus adiabatic descent does NOT involve transfer of heat or energy from the surrounding gases. The surrounding gases do NOT cool or become less energetic as heat is created within the descending air.
The heating in adiabatic descent comes from WITHIN the descending parcel via conversion of gravitational potential energy to kinetic energy. The total amount of energy in each molecule remains the same but it changes form with uplift and descent.
It is true that the descending parcel does work on the surrounding gravitational field but there is no transfer of heat or energy from the surrounding gases.
Furthermore, the potential energy created during ascent does not escape to space. It is stored within the parcel as gravitational potential energy which is not heat and therefore cannot radiate away.
It is then returned as heat on the descent and ADDS to the heat generated by the flow of solar radiation passing through the system. That is why it raises the temperature above S-B by 33C.
I think you have the idea that non heat energy passes from the surrounding gases during adiabatic descent and that the energy so transferred to the descending parcel causes it to warm.
That is not what happens. In an adiabatic process there is no net transfer of either heat or energy to or from the surrounding gases. The work done by the ascending or descending parcel is done with or against gravity so that the surrounding gases are unaffected.
Movement within a gravity field does not involve any addition or removal of energy. It just causes the energy already present to change form between kinetic energy (heat which radiates) and potential energy (not heat which does not radiate).
Tim Folkerts said:
“There will be NO (net) convective loss on such a planet with a transparent atmosphere! The net lose from the atmosphere to space is zero. The net input to the atmosphere from the sun is zero. Hence the net input to the atmosphere from the ground must ALSO be zero. There would be some convective transfer from surface to atmosphere during the day, and some convective transfer back from atmosphere to surface during the night. But the net result is zero. ”
There is the error.
As long as there is any convection there will be surface kinetic energy being converted to gravitational potential energy (GPE) higher up and the latter cannot be radiated to space until it returns to the surface or leaks out to space from radiative gases, aerosols or condensate within the atmosphere.
There must always be convection from an unevenly heated surface due to that uneven heating causing density and weight variations in the horizontal plane. GHGs are not necessary for convective overturning, only uneven conduction in the horizontal plane between surface and atmosphere.
If there are no GHGs then ALL that GPE has to be returned to the surface before it can be radiated to space. In reality it doesn’t warm the surface directly but rather reduces the rate of surface cooling which on Earth causes the surface to rise 33C above the S-B prediction in order to restore radiative equilibrium with space.
If the atmosphere were 100% radiative then none of that GPE would need to be returned to the surface for radiation to space because it would all leak out directly from within the atmosphere.
It is the radiative atmosphere that has weaker convective overturning because less energy goes back down in adiabatic descent than is taken up during adiabatic ascent. Radiative leakage to space from within the atmosphere causes the difference.
There may be no net convective loss (via radiation from within the convective column) to space from a planet with a transparent atmosphere but then the convective overturning cycle must be MORE powerful to return GPE to the surface fast enough to ensure that radiation from the surface alone matches radiation in from space.
Stephen Wilde says: September 15, 2014 at 11:17 am
Kristian said: (“Once again, no, Stephen. Descending air warms adiabatically WITH an addition of new energy, only not HEAT. That’s what ‘adiabatic’ means. For the hundredth time. The energy it delivered to the surrounding atmosphere on ascent it gets back on descent. The energy is transferred across the adiabatic barrier in the form of WORK – expansion going up, compression coming down.”)
“Kristian, most of what you say is correct but that bit is not and it skews the rest of your interpretation of reality.”
“An adiabatic process is a process that occurs without the transfer of heat or matter between a system and its surroundings.”
Very good Stephen. Now you are expressing what can be observed and measured ( the physical) rather than some fantasy. OTOH,”Who”, exactly, has any concept of reality? I must stick with the physical, and hang on dearly. Kitten agrees!
Just out of interest I used equation 107 from page 70 of G&Ts paper
Click to access 0707.1161v4.pdf
This was to contrast the radiative loss from a bottom face of a cubic metre of dry air with the radiative loss from the top face of a cubic metre of water.
This is the most common interface on our planet covering almost 70% of the surface area.
For both the calculated temperature drop is a modest one unit from 300K to 299K
The loss of internal energy would be typical for night time conditions
For air answer is 2 milliseconds
For water it is 2.54 hours
We don’t even need to invoke the second law to note that radiative warming of the surface by the atmosphere is pretty far fetched.
Will 5:18am: “This is Trick’s fantasy. In an open system such as Earth and its atmosphere any application of 1st law is false.”
Every system in the universe is open. The only closed system expected to exist is the universe as a whole.
In thermodynamics, the 1st law is invoked through the use of a control volume across which energy entering and exiting any arbitrary open system is accounted for and the change in internal energy thus computed or measured.
Since Will challenges the 1st law as faulty, meaning that energy can be created from nothing and even destroyed with nothing left over, Will has a big challenge to prove the hypothesis. If Will chooses to challenge the 2nd law, there is nope for Will.
“The, Line By Line Radiative Transfer Model is but a “deliberate” corruption of all the carefull measurements contained in the USAF HiTran data base.”
No. The line by line transfer model & HITRAN agree to within instrumental error as shown by the papers I cited at 2:54pm and many more. This is not disputed in science anymore Will – since 1995 paper in the top post.
“Can you give any possible definition to your use of the word “diffraction” on the moon?”
See Max Planck’s original paper definition on the subject – you know the one that made known the Planck distribution. If you can’t find it – just ask.
“Only Trick would claim that adding dispersive matter to a gas would increase optical depth. All others would agree that such addition must decrease optical depth.”
Shine a beam of light thru a gas – add dispersive matter – count less photons in the beam upon exitance, as more photons are dispersed with increased optical depth.
“Correct LBLRTM analysis must only confirm convection!”
LBL Radiative transfer + 0 convective transfer = top post picture
Radiosonde data + convective transfer = top post picture
I’ll let Will do the math to find the amount of convective transfer in top post picture.
“…an increase of lower temperature….No thermodynamic transfer involved.”
Here Will postulates an increase of temperature without energy transfer. This is why Will disputes the 1st law. Good luck Will.
Kristian 7:40am: “You can deny this until you’re blue in the face, Trick. It is still true.”
Then I’m blue in the face. The classic text book example proving Kristian is wrong about “The mass of the atmosphere, however, clearly does. By being able to warm (‘heat capacity’). And by exerting pressure on the surface (weight).” is shown in Fig. 1.3 Bohren 1998 text p. 18.
A weight is added to a piston over a container of gas. There is mass and heat capacity at equilibrium but the gas is at room temperature, no warming.
“Shine a beam of light thru a gas – add dispersive matter – count less photons in the beam upon exitance, as more photons are dispersed with increased optical depth.”
That would be decreased optical depth as Will says.
Increased optical depth means that photons travel further without interference i.e more transparent / less opaque.
More dispersion is decreased optical depth because photons travel less far before suffering interference.
“Will postulates an increase of temperature without energy transfer”
Energy can transform within a parcel of gas from potential (a form of latent) energy (not heat) to sensible energy (heat) and back again without any transfer of energy between the parcel and its surroundings. That is the essence of adiabatic warming and cooling.
Trick gets a lot of other stuff wrong too so I’ve been ignoring him.
Stephen 2:44pm: “That would be decreased optical depth as Will says. Increased optical depth means that photons travel further without interference i.e more transparent / less opaque.”.
Stephen – Less photons in the beam going out means increased optical depth in all the relevant text books. Since Stephen hasn’t bothered to get the pre-req.s to read them, it is easy for Stephen to miss this point. All my stuff is simply text book physics which Stephen hasn’t consulted. Stephen being unencumbered by physical law, is free to imagineer like the Disney Co. except without their blending in engineering.
@Kristian, September 15, 2014 at 7:53 am
I just noticed that I referrred to Nikolov & Zeller as “N&K”. Ooops…..some kind of Freudian slip?
My take is that the R&C model degenerates into a lapse rate of -g/Cp as predicted via thermodynamics when the pressure exceeds one bar. This is because energy transfer by convection increases as pressure rises while the distance over which radiation operates falls. The title of R&C, 2014 says it well:
“Common 0.1 bar tropopause in thick atmospheres set by pressure-dependent infrared transparency”
In Earth’s atmosphere the 15 micron “back radiation” one can measure at the surface is coming from the first 100 meters of the atmosphere. At high pressures gases like CO2 absorb more radiation due to collision broadening but most of the extra energy absorbed is given up to surrounding molecules before it can be re-radiated.
I was hoping you would be impressed by the excellent “fit” achieved by the R&C model for the HASI probe data (Titan). In my opinion that is a tremendous achievement.
Stephen: “Energy can transform within a parcel of gas from potential (a form of latent) energy (not heat) to sensible energy (heat) and back again without any transfer of energy between the parcel and its surroundings. That is the essence of adiabatic warming and cooling.”
Concur. No change in Tmean of Stephen’s parcel. Will just wrote the parcel mean temperature can increase in this case. This means Will postulates the 1st law is faulty. Good luck Will.
Stephen – Standard simple parcel mechanics disallow (rule out) the condensation inside for DALR but as long its energy is properly accounted for as in nature, a more detailed analysis will prove 1st law.
@Tchannon: As I said: No worries. Thanks for the link!
On of my ‘problems’ is too little pridefulness or sense of ownership. All I really care about is that ‘truth be known’. And it is kind of nice to see an idea being useful to others.
@GallopingCamel & Tom0Mason: Thanks for the endorsements 😉
@All:
I see that once again the bogosity of “Average Global Temperature” is causing arguments. There is not and can not be an “average global temperature” that has any meaning. As an average of an “Intensive Property”, it is void of meaning. ALL radiative heat transfer is based on the unique LOCAL temperatures. ANY use of TAVE in radiative transfer will give bogus results for our planet.
Per the assertion the authors of the paper think CO2 will cause warming: Note the restrictions they put on that part. Reduce the contribution from water vapor, in the radiative mode. Now is that as a percent, or a total? As water vapor almost entirely matters in the convective (non-radiative) troposphere, it is a fancy way of saying “will not do much”… To paraphrase “CO2 will do some more radiating (that will be absorbed again) so water vapor will be doing less of the total, in that part of the air where convection an enthalpy are all that really matters; and this is consistent with things gaining energy (or not).”
Per tropopause height: It isnt fixed. Varies by latitude, by season, by time of day. Individual convective cells can punch ‘through’ (higher) by 10s of Thousands of feet. It is exactly the place where convection has finished moving (however much heat was to be moved) to the radiative zone where CO2 is a net radiator.
Per that adiabatic issue: There IS energy flow, just not heat flow. Decending air is turning potential energy of height (gravity) into thermal (compression). Rising air turns thermal into potential doing work against gravity. There is energy exchange going on, just not heat flows (thremal to thermal).
Hopefully some of this helps.
gallopingcamel said:
“Common 0.1 bar tropopause in thick atmospheres set by pressure-dependent infrared transparency”
That sounds rather as though it is mass density that controls the transparency of an atmosphere to infrared.
If so, that would suggest that it is conductive (rather than convective or radiative) energy transfer from mass to mass that heats the atmosphere.
Radiative fluxes are then relegated to merely being a consequence of the energy distribution (temperature along the lapse rate slope) set up by conduction and density rather than a cause of that temperature.
Perhaps you should say:
“energy transfer by CONDUCTION increases as pressure rises while the distance over which radiation operates falls.”
The increase with pressure being a result of the fact that greater density allows more conduction from a given amount of solar energy flowing through.
Greater density allows more conduction but less radiation due to the increased opacity to infrared radiation.
As conduction rises, radiative transfer reduces but both must always add up to the amount of energy coming in from space if the atmosphere is to be retained.
Hence my contention that the more radiative an atmosphere the less radiation there can be from surface to space.
Radiation from the surface to space varies inversely to radiation from the atmosphere to space in order to maintain equilibrium and as a by product of that principle GHGs cannot result in a warmer surface because increased radiation from within the atmosphere reduces energy returning to the surface in adiabatic descent.
E M Smith said:
“Per that adiabatic issue: There IS energy flow, just not heat flow. Descending air is turning potential energy of height (gravity) into thermal (compression). Rising air turns thermal into potential doing work against gravity. There is energy exchange going on, just not heat flows (thermal to thermal).”
Agreed, thank you.
And after the first convective cycle closes the adiabatic loop one is left with a surface temperature 33C higher than the S-B prediction AND total radiative loss from surface plus atmosphere equal to incoming from space.
Stephen says: “There is the error.
As long as there is any convection there will be surface kinetic energy being converted to gravitational potential energy (GPE) higher up and the latter cannot be radiated to space until it returns to the surface or leaks out to space from radiative gases, aerosols or condensate within the atmosphere.”
I was specifically talking about a transparent atmosphere. I agree that “the latter cannot be radiated to space until it returns to the surface or leaks out to space from radiative gases, aerosols or condensate”, but the hypothesis of a transparent atmosphere says that ” leaks out to space from radiative gases, aerosols or condensate” do not occur. Thus the energy cannot be converted to radiation until it returns to the surface. So all energy that goes up into the atmosphere would have to return to the surface in this case.
Thus there can be no net transfer to the atmosphere. And the surface loses net energy only via radiation directly to space, so the surface will be somewhere around 255-270 K.
Tim,
You accept that a transparent atmosphere in which convection occurs must return energy to the surface.
Do you accept that the surface will then be receiving both continuing insolation PLUS energy being returned in the descent ?
Why do you not accept that those two sources of energy added together will raise surface temperature 33C above the S-B prediction ?
The 33C warmer surface will still not radiate more to space than S-B expects because the energy represented by that 33C surface temperature enhancement goes straight back to the atmosphere in the next adiabatic ascent.
The bit you are missing is that adiabatic uplift is indeed an energy transfer from surface to atmosphere. Energy conducted to the air is converted in uplift to gravitational potential energy which results in cooling along the lapse rate slope.. Such energy is removed from the radiation budget into the separate adiabatic loop and has no effect on temperature whilst in potential form. That removed energy does not cool the surface below the S-B temperature since it is taken not from the surface but from the flow of radiation to space after it has left the surface at the S-B rate.
During the first convective cycle a view from space would appear to show a cooler surface but the surface is not cooler. Instead the atmosphere is absorbing some of the outgoing radiation to give the external viewer a false impression.
That happens with or without GHGs in the atmosphere due to uneven surface heating.
Once the first convective cycle completes there is no longer any net energy transfer between surface and atmosphere but during the first cycle there has been a net conversion of energy, that would have otherwise been radiated to space, to gravitational potential energy.
That pool of energy which accumulates in the very first adiabatic convective cycle is what raises surface temperature for ever after.
It forever thereafter warms the surface on descent but still fails to escape to space because it is being constantly recycled in the adiabatic loop.
The radiative fluxes are then a consequence of those mechanical processes which involve work done with and against gravity.
GHGs just alter the size or speed of the adiabatic loop without affecting surface temperature because whatever they allow to leak out to space reduces the amount of energy returned to the surface on descent by exactly the same amount.
Energy cannot be in two places at once.
“Do you accept that the surface will then be receiving both continuing insolation PLUS energy being returned in the descent ?</i"
That is a good start.
The surface receives power at some average rate P_sun-to-surface from the sun. The surface loses power to the atmosphere at some average rate P_surface-to-atmosphere from the warm parts of the surface. The surface gains power at some average rate P_atmoshphere-to-surface. The surface also loses power straight to space at a rate P_surface to space.
The average power to the surface is
P_in = P_sun-to-surface + P_atmosphere-to-surface
The average power leaving is
P_out = P_surface-to-atmosphere + P_surface to space.
The NET power to the surface is then
P_in – P_out = P_sun-to-surface + P_atmosphere-to-surface – P_surface-to-atmosphere – P_surface to space
“Why do you not accept that those two sources of energy added together will raise surface temperature 33C above the S-B prediction ?”
Because the Net Power can be rewritten as
P_in – P_out = P_sun-to-surface + (P_atmosphere-to-surface – P_surface-to-atmosphere) – P_surface to space
And P_atmosphere-to-surface = P_surface-to-atmosphere, so this simplifies to
P_in – P_out = P_sun-to-surface + (0) – P_surface to space
Or P_sun-to-surface = P_surface to space
Basically all of that math simply says that any energy returned to the surface from the atmosphere must have come from the surface to begin with. The net effect is no warming or cooling. The Surface is the same temperature as it would have been without the transparent atmosphere.
Trick said:
“All my stuff is simply text book physics”
I don’t accept that. The text book physics that I have seen differs from Trick’s interpretations.
Anyway, Trick should think from first principles if he wants to get it right and not simply parrot misunderstood and incomplete bits of the physics that he prefers.
“During the first convective cycle a view from space would appear to show a cooler surface but the surface is not cooler. Instead the atmosphere is absorbing some of the outgoing radiation to give the external viewer a false impression.”
No. If the atmosphere is transparent, the surface really WILL be cooler. A transparent atmosphere cannot absorb any radiation and cannot give a false impression.
If the atmosphere is NOT transparent, then it can absorb and can give a “false impression” of the surface temperature. But that is exact what the GHE is all about.
Tim said:
“Basically all of that math simply says that any energy returned to the surface from the atmosphere must have come from the surface to begin with. The net effect is no warming or cooling. The Surface is the same temperature as it would have been without the transparent atmosphere.”
Then you’ve got it wrong.
The energy that is returned to the surface did indeed come from the surface initially but its escape to space was delayed by the conductive/convective exchange within the atmosphere.
Therefore, after the first convective cycle completes it must have a warming effect at the surface.
It is a timing issue in a situation where there is a constant throughput of new energy from the sun.
Tim said:
(i) “A transparent atmosphere cannot absorb any radiation and cannot give a false impression.”
It can absorb by conduction and convection and therefore can give a false impression during the first convective cycle when viewed from space. Conduction is just radiation diverted to a mechanical process before it escapes the atmosphere.
The view from space will also be distorted during any period of imbalance between conduction / convection and radiation but the balance is always restored by a change in convection.
Energy absorbed into conduction and convection is no longer available for radiation to space because it converts to potential energy which cannot radiate.
(ii)”But that is exactly what the GHE is all about.”
I agree but it is due to mass absorbing by conduction and then convecting up and down. Nothing to do with radiative gases.
(iii) “If the atmosphere is transparent, the surface really WILL be cooler”
Not in the presence of conduction and convection it won’t. And you cannot eliminate conduction and convection by removing GHGs because there is still plenty of uneven surface heating in the horizontal plane causing density and weight variations in the gases above the surface.
Anyway, a transparent atmosphere allows sunlight in at maximum power. How would that lead to a cooler surface ?
I suppose you would say that the actual atmosphere is more transparent to incoming than it is to outgoing so adding GHGs transmits more radiation to the surface to add to that arriving from space.
Unfortunately if that were true you would be double counting the energy returning to the surface since you have already accepted that energy is returned to the surface by descending air.
I think you and radiative theory have a real problem there 🙂
E.M.Smith 3:28pm: “Per that adiabatic issue: There IS energy flow, just not heat flow. Decending air is turning potential energy of height (gravity) into thermal (compression). Rising air turns thermal into potential doing work against gravity. There is energy exchange going on, just not heat flows (thremal to thermal).”
In the adiabatic parcel mechanics, enthalpy is the parcel’s conserved quantity. There is NOT energy exchange across the control volume of the adiabatic parcel (by definition!). This means internal energy of the adiabatic parcel remains unchanged on ascent and descent by 1st law. As the parcel ascends, sure, its internal KE is changed to PE meaning parcel temperature mean drops and p*V term adjusts to conserve enthalpy. On descent, PE is changed back to KE, parcel Tmean rises and p*V term again adjusts to keep constant enthalpy. All with constant internal energy.
I will write that again, it is important: All with constant internal energy meaning constant parcel enthalpy. No energy exchange outside the adiabatic parcel control volume. No means: no exceptions.
This process means no matter outside the control volume is cooled or warmed by the parcel at any altitude at any time at any freq. including & especially z=0, the surface. The surface Tmean remains unaffected by adiabatic parcel ascent and descent – as shown by the top post analysis. Stephen has never been able to grasp this concept or even grasp the p*V term mechanics. Because he has not the modern pre-req.s to read a relevant modern text.
This adiabatic process assumption results in about a 20% difference in lapse from environmental lapse up to tropopause. Poisson relaxed the adiabatic constraint somewhat, let T vary ideally and found the ideal, exact lapse off less, only about 10% from measured. This over 100 years ago. So it was available for Stephen’s study in the 1960s yet he cannot demonstrate a grasp of the concepts.
Stephen 8:15pm: “I don’t accept that. The text book physics that I have seen differs from Trick’s interpretations.”
I already know asking for a cite to check will do no good Stephen. Last time I went to check on this, Stephen could not provide a text title, text author, publish date, or any specifics at all.
“Trick should think from first principles…”
Noted, thank a teacher today. All my comments from text books are traced within them back to 1st law and Planck’s distribution relevant on this thread, precise cites provided if asked. Stephen hasn’t accomplished the pre-req.s to actually read the texts to find that out. Stephen prefers not to do the requisite reading in order to remain comment free from compliance with most if not all natural laws.
Stephen 8:45pm: “I agree but it is due to mass absorbing by conduction and then convecting up and down. Nothing to do with radiative gases.”
The top post analytical picture produced entirely from radiation once compared to radiosonde test data producing the same picture proves this wrong.
Trick says:
“This process means no matter outside the control volume is cooled or warmed by the parcel at any altitude at any time at any freq. including & especially z=0”
That is true only AFTER the first convective cycle has completed.
It is NOT true at the moment the first convective cycle closes the adiabatic loop.
At that point, kinetic energy returning to, or arriving just above, the surface reduces the rate of surface cooling by a specific amount related to the size and speed of convective overturning.
Whilst the adiabatic loop remains open during the first convective cycle there is a net transfer of kinetic energy to gravitational potential energy from the radiation leaving to space from the surface which remains at the S-B temperature and once the first convective cycle completes the thermal effect lasts for as long as the atmosphere remains suspended off the surface.
On Earth, that means that the surface temperature is enhanced by 33C.
Trick and others are trying to argue that the adiabatic loop is closed whilst the first convective cycle remains incomplete.
They are wrong.
Stephen 10:18pm – The parcel is defined adiabatic in the very first cycle also. No exceptions.
Trick said:
“The parcel is defined adiabatic in the very first cycle also. No exceptions.”
Your point was that an adiabatic process is net zero because energy taken up is balanced by energy taken down.
That is not the case during the first cycle.
After the first cycle the energy arriving back at the surface must be added to continuing solar input for a surface temperature enhancement of 33C.
“The top post analytical picture produced entirely from radiation once compared to radiosonde test data producing the same picture proves this wrong.”
It ‘proves’ no such thing.
It merely shows that the real world energy distribution is far more complex than the basic principles would suggest because a multitude of other factors intervene.
But, in the end, all those complications are smoothed out by the convective response and the system remains stable.
Although its seems counterintuitive trying to calculate values of air temperature at various heights based on kinetic energy and potential energy of molecules will not give you the correct answer.
A useful visual analogue is the Cartesian Diver ascending through water.
It gains PE without losing KE all because the air bubble (included) expands doing work against the surrounding water pressure.
Similarly an ascending air parcel only needs to be slightly warmer than its surroundings and because of lower density moves up.
It expands doing work AGAINST the surrounding air, losing internal energy (temperature drops) losing energy from the parcel (this energy is transferred to its surroundings).
Similarly a descending air parcel only needs to be slightly colder than its surroundings and because of lower density moves down.
The surrounding air then does work ON the parcel compressing it and hence increasing its internal energy (temperature increases) ( this energy is transferred FROM the surroundings to the parcel).
Correction
Should read
Similarly a descending air parcel only needs to be slightly colder than its surroundings and because of HIGHER density moves down.
Stephen 10:30pm: “That is not the case during the first cycle.”
Sure it is, no exceptions: the 1st amount of energy in the very 1st adiabatic parcel went up, was conserved, came down the same, no energy exchanges, enthalpy conserved. No exceptions. 1st law is good all the time even 1st cycle, everywhere, at all temperatures.
10:36pm: “(Top post picture) merely shows that the real world energy distribution is far more complex than the basic principles would suggest because a multitude of other factors intervene.”
Not at all. This merely shows Stephen’s lack of being able to read the science about the mechanics of the top post picture. The proof is simple. The top post analytical picture produced entirely from radiation once it is compared to radiosonde test data that produces the same picture!
Think about that Stephen: radiation entirely produces that top post picture, natural convection had no effect on its production. Test including natural convection produces the same picture. This is why the gov. can produce IR pictures of convective storms, of an object within the atm. without worrying about windy days, IR goggles work on windy days.
Concur the system is approx. stable with small anomaly. Guess I am not on Stephen’s ignore after all.
Stephen says “That is true only AFTER the first convective cycle has completed.”
But until the first cycle has completed, convection is cooling the surface. Any “warming” is merely going to try to make up for the cooling that has already occurred. This “returned enenrgy” will be warming the surface BACK UP to where it would have been if the energy had not been “stolen ” to begin with.
Trick says: September 15, 2014 at 2:14 pm
Will 5:18am:( “This is Trick’s fantasy. In an open system such as Earth and its atmosphere any application of 1st law is false.”)
“Every system in the universe is open. The only closed system expected to exist is the universe as a whole.In thermodynamics, the 1st law is invoked through the use of a control volume across which energy entering and exiting any arbitrary open system is accounted for and the change in internal energy thus computed or measured. Since Will challenges the 1st law as faulty, meaning that energy can be created from nothing and even destroyed with nothing left over, Will has a big challenge to prove the hypothesis. If Will chooses to challenge the 2nd law, there is nope for Will.”
I did not challenge any thermodynamic law! The 2nd is a definition of a spontaneous process and applies universally. The 1st applies only to a closed thermodynamic system where entropy collects as unuseful energy. It is only your blatent misapplication of the Laws that is false.
(“The, Line By Line Radiative Transfer Model is but a “deliberate” corruption of all the carefull measurements contained in the USAF HiTran data base.”)
“No. The line by line transfer model & HITRAN agree to within instrumental error as shown by the papers I cited at 2:54pm and many more. This is not disputed in science anymore Will – since 1995 paper in the top post.”
The correct name is Line By Line Radiative Transmission Model. It has nothing to do with any power or energy transfer. It only has to do with the atmospheric attenuation of an identifiable signal “not in radiative equilibrium” with that air mass. The corruption is the deliberate attempt to apply that model to flux attenuation in an atmosphere that remains mostly “in radiative equilibrium”.
Your cited papers have no relevance: 1. CFCs and HFCs only so what? 2. “Downwelling thermal infrared emission from the tropical atmosphere”. Huh! Demonstrate any ever? Later same paper measured spectral radiance (not emission) from 5 to 20 microns. Which radiance or emission?
3. Graph of zenith specular radiance (notice the per steradian on the left). In that 14.5 micron CO2 band at the lower apparent temperature of the tropopause the exit steradiance is twice that of any possible surface radiator. Same happens for the 9.5 micron O3 band
(“Can you give any possible definition to your use of the word “diffraction” on the moon?”)
“See Max Planck’s original paper definition on the subject – you know the one that made known the Planck distribution. If you can’t find it – just ask.”
So you cannot yourself define the words you write. I know the meaning of defraction. It dos not happen on the moon. Perhaps you ment scattering.
(“Only Trick would claim that adding dispersive matter to a gas would increase optical depth. All others would agree that such addition must decrease optical depth.”)
“Shine a beam of light thru a gas – add dispersive matter – count less photons in the beam upon exitance, as more photons are dispersed with increased optical depth.”
“Optical depth” is precisely that distance through a medium where transmittance is (1-1/e).
(“Correct LBLRTM analysis must only confirm convection!”)
“LBL Radiative transfer + 0 convective transfer = top post picture
Radiosonde data + convective transfer = top post picture”
“I’ll let Will do the math to find the amount of convective transfer in top post picture.”
What nonsense! The illustration has only partial specular radiance of Le Chatelier’s Principle in the atmosphere. It has nothing to do with LBL anything.
[“…an increase of lower temperature….No thermodynamic transfer involved.”] fake!
(“What total nonsense. Gravitational attraction alone compresses the N2 and O2 in the lower atmosphere resulting in an increase of lower temperature. This is a thermostatic pressure and temperature gradient both increasing at lower atmosphere No thermdynamic energy transfer is involved.”) origional!
“Here Will postulates an increase of temperature without energy transfer. This is why Will disputes the 1st law. Good luck Will.”
I take it you have no concept of thermostatic state in a gravitational field!! Thermodynamically, the adiabatic part of the troposphere is an isotherm with high thermal conductivity, and all pressure and temperature gradiants mantained by gravitational attraction of a compressable gas. No process, no energy transfer.
Tim Folkerts says: September 15, 2014 at 11:51 pm
Stephen says “That is true only AFTER the first convective cycle has completed.”
“But until the first cycle has completed, convection is cooling the surface. Any “warming” is merely going to try to make up for the cooling that has already occurred. This “returned enenrgy” will be warming the surface BACK UP to where it would have been if the energy had not been “stolen ” to begin with.”
What wierd thinking! No “heat energy” transfered to a lower temperature atmosphere, is ever returned to the higher temperature surface by any means whatsoever.
Tim, I know you and I differ in the word “heat”. I use “heat energy” to distinguish that part of internal energy, that at “sometime” may become both temperature to create radiance, and the sensible heat that powers any radiant exit flux, and maintains that temperature. Both are required for any thermal EMR exitance, Mass x velocity x velocity/2 does not radiate.
Consider water, evaporation is a endothermic process that creates a gas with 2300 Joules per gram of latent heat. This new gas having very low density is transported upward by hydrostatic equilibrium, with or without work. At a lower temperature altitude this gas condenses, highly exothermic 2500 J/g of sensible heat but cold and can do no work. That sensible heat is that work/local temperature, (entropy).
Because of that temperature the remaining WV has “radiance”, a potential for EMR entropy transfer in any direction toward a lower temperature (space). For each WV molecule having only cross sectional area and quite sparce in the atmosphere, each WV molecule is free to radiate equally into a whole very low radiance hemisphere at all wavelengths from 14 to 200 microns. This is where all of the exit flux from this planet originates.
Please marvel at the competence of designers and constructors of this earth. Even if this planet did its own design and construction, the competence is very evident. Earthlings are mearly the current top predator. How wonderful!! Roaches may be next, they seem immune from ebola!
Trick says
“Think about that Stephen: radiation entirely produces that top post picture, natural convection had no effect on its production.”
This is a computer model simulation not a real physical event.
Trick should think about the fact that the barometric formulas give detailed accurate temperature, pressure and density profiles for the troposphere without any reference to separate radiative transfer calculations.
Now why is that?
Because the radiative properties of (say) air are already included in the bulk thermodynamic quantities such as specific heat capacity.
Of course if Trick can point me to a table of say the constant volume heat capacity of carbon dioxide at various temperatures with the radiative content stripped out I will change my opinion.
As soon as you think about it you realise that an experiment to determine such a table would be impossible to carry out.
The thermodynamic properties are inextricably linked!
Tim said:
“But until the first cycle has completed, convection is cooling the surface”
Conduction and convection do not cool the surface below the initial S-B temperature.
Instead, they take energy from the outgoing radiation with no effect on the rate of cooling of the surface.
In order to reduce the surface temperature below S-B the processes of conduction and convection would have to operate faster than radiation but they clearly do not.
So, surface temperature remains at S-B during the first cycle but then takes a 33C jump up when the first cycle completes and it stays elevated for as long as the atmosphere remains suspended off the surface.
Bryan,
A rising parcel of air in cooling does not warm the surrounding molecules so it is incorrect to say that it is transferring energy to those molecules. Instead it maintains its internal energy but converts an increasing proportion of it from kinetic to potential energy.
Trick said:
“the 1st amount of energy in the very 1st adiabatic parcel went up, was conserved, came down the same, no energy exchanges, enthalpy conserved. ”
That first amount of energy was removed from the surface INSTEAD OF radiating to space. It then returned to the surface IN ADDITION to continuing insolation.
That results in a net addition to the on going surface energy budget worth 33C.
It is a timing issue. There is an accumulating imbalance until the first cycle completes and then there is a sudden surface temperature adjustment to retain overall equilibrium.
Stephen Wilde says
“A rising parcel of air in cooling does not warm the surrounding molecules so it is incorrect to say that it is transferring energy to those molecules. Instead it maintains its internal energy but converts an increasing proportion of it from kinetic to potential energy.”
Think of a parcel expanding to the left and molecules travelling from the left meeting like a bat and ball.
The ball will come off with slightly increased KE or temperature increase.
Now what you are saying is fairly plausible but what you must ask yourself ‘does it correspond to reality?’
Theres an easy way to find out.
Your theory is the loss of internal energy on ascent is turned into potential energy.
So take a mole of air at STP.
Work out its internal energy from formula KE =Nk x1.5T
N = Avogadro’s Number
k = Boltzmann’s Constant
T =Standard Temperature in Kelvin units = 273K
Now do the same at a height of one Kilometre with the same formula but with T2 = T -9.8
The 9.8 comes from the drop in temperature due to adiabatic lapse rate drop for one kilometre
Subtract this value of internal energy from the STP value.
Now if you are correct this value will be equal to the gravitational potential energy gained.
PE = mgh
m = mass of one mole of air in kilograms
g = 9.81
h = 1000
All with the correct units implied
I think you will find that the energies will not equal one another.
To get the correct answer you need the barometric formulas for an adiabatic atmospheric expansion.
Page 8 of this link
Click to access 1003.1508.pdf
Bryan,
You seem to be limiting your consideration to the kinetic energy of linear movement as in a bat hitting a ball.
The kinetic energy of a molecule that converts to gravitational potential energy is its vibrational movement. All molecules vibrate all the time as long as they are warmer than absolute zero.
Molecules that vibrate faster emit more infra red radiation and are sensed as warmer.
Molecules that vibrate slower emit less infra red and are sensed as colder.
A molecule under pressure at the bottom of an atmosphere is experiencing the force of gravity seeking to compress the components of the molecule into a smaller space. That results in smaller but faster vibrations, emission of more IR and a higher sensible temperature.
As pressure is released with height the vibrations become larger but slower, emission of less IR and a lower sensible temperature.
The total internal energy remains the same but it is reapportioned between sensible energy (heat) and non sensible (potential) energy.
I’ve seen a lot of commenters thinking that kinetic energy is just that which is involved in linear movement. That misapprehension leads them to think that the potential energy numbers are too small to produce the observed result of adiabatic warming on descent which is a large effect.
In fact, the gravitational compression and decompression of the molecules in atmospheric gases makes a big difference to the emission of IR between the top of our atmosphere and the surface.
Note, though, that since our standard atmosphere (STP) is partly radiative not all vibrational kinetic energy is retained as potential energy. A portion is radiated out to space (leakage from the adiabatic process) so the numbers will not be equal.
This is basic physics from years ago, not something I just made up.
Bryan 8:32am: “This is a computer model simulation not a real physical event.”
Concur. A computer model picture built from line by line radiative transfer model only. The radiosonde data from real physical events includes the effects of your “barometric formulas” yet produce the same picture so “barometric formulas” are of no consequence to the picture.
1:30pm: The formulas you refer (p.8) were worked out by Poisson over a century ago. They result in the ideal, exact lapse rate which is closer to reality than DALR.
******
Stephen 9:36am: “That first amount of energy was removed from the surface INSTEAD OF radiating to space. It then returned to the surface IN ADDITION to continuing insolation.”
Energy cannot be in two places at once; the first adiabatic parcel contained convective energy ascending then descending that did NOT radiate to space, stayed in atm. Other energy radiated to space just like the accounting of today. However the numbers were different in that epoch.
******
Will 6:24am: What you write is irrelevant nonsense (aka gibberish) of your own concoction with no foundational cites at all. Please fill me in on diffraction with cite as defined by Max Planck (not Will) and why you propose it is not present in the moon including foundational citations. For LBLRTM definition see my cites at 2:54pm:
“We used the line-by-line radiative transfer forward model LBLRTM [Clough et al., 1992; Clough and Iacono, 1995] (version 5.4) to generate atmospheric spectra…”
“This model subsequently has been incorporated into several widely distributed radiative transfer codes (LBLRTM, MODTRAN…Model residuals are on the order of the uncertainty in measurements,especially of the atmospheric water vapor and temperature profiles.”
Stephen 2:07pm: “This is basic physics from years ago, not something I just made up.”
Until you provide the exact cite from years ago, it IS something you just made up.
“the first adiabatic parcel contained convective energy ascending then descending that did NOT radiate to space, stayed in atm”
Yes it did but in the process reduced surface cooling to space as soon as the first cycle completed and closed the loop. It then caused the 33C surface temperature enhancement.
Stephen 4:17pm: “Yes it did..”
No. 1st adiabatic parcel did NOT radiate to space from within. No energy of any kind crossed that 1st adiabatic parcel boundary upon ascending or descending – this is the meaning of adiabatic. This should be simple to understand but Stephen demonstrates cannot grasp the foundational science. The 1st parcel cannot cause the 33K as demonstrated by the top post picture analysis compared to radiosonde test – no meaningful difference in radiative transfer analysis and test observed.
Stephen says: “Conduction and convection do not cool the surface below the initial S-B temperature.
Instead, they take energy from the outgoing radiation with no effect on the rate of cooling of the surface. “
Not if the atmosphere is transparent! An atmosphere that is transparent to IR by definition cannot take energy from the outgoing IR. Now it the atmosphere is NOT transparent to IR, then the what you say sounds pretty much right — but again, now we are back to the “normal” GHE.
As usual, Trick, you misread English.
I was simply agreeing that the energy did stay in the atmosphere, not asserting that it radiated to space.
Sheesh.
Since it stayed in the atmosphere it reduced the rate of cooling of the surface beneath which then had to rise by 33C to restore radiative equilibrium with space.
Stephen
I must apologize for giving the wrong formula up thread.
Instead of the 1.5 factor it should be 2.5 because air is 99% diatomic and has 5 degrees of freedom instead of the usual three translational.
Anyway your theory does not give realistic answers.
Try and work them out for yourself
check your answers against mine
Internal Energy lost = 203J
Potential Energy at 1000m = 284.2J
Work done in adiabatic expansion using the barometric formula’s = 203J
That why your method (although quite plausible) does not work
Tim said:
“Not if the atmosphere is transparent! An atmosphere that is transparent to IR by definition cannot take energy from the outgoing IR. Now it the atmosphere is NOT transparent to IR, then the what you say sounds pretty much right — but again, now we are back to the “normal” GHE.”
The mass of the atmosphere takes energy INDIRECTLY from the outgoing radiation by absorbing surface energy by conduction INSTEAD of it radiating to space.
By enabling conduction mass alone can take energy away from the outgoing IR. The same parcel of energy cannot be in two places at once. It must either leave the surface by radiation OR by conduction.
The energy leaving from the surface at the normal S-B rate is then split between radiation to space and conduction to the air.
You can’t have energy leaving the surface faster than it comes in. The surface cannot go lower than S-B unless conduction and convection acts faster than radiation but it does not. It is much slower.
If conduction to atmospheric mass could reduce the surface temperature below S-B then the more massive the atmosphere the colder the surface would be. That is the opposite of the truth.
Surface temperature stays the same as the S-B temperature despite conduction but only until the first convective cycle completes the loop whereupon the surface is then receiving the kinetic energy returned to the surface on descent PLUS continuing insolation at the original rate.At that point Earth’s surface temperature rose 33C above S-B.
It was a result of conduction and not radiation.
You could see the point if you were not emotionally biased against it.
Bryan,
If your numbers are right please explain adiabatic warming on descent at the dry adiabatic lapse rate.
On the face of it, your figures purport to prove that adiabatic warming and cooling are impossible.
Good luck with that 🙂
The kinetic heat energy acquired by the descending air is not being drawn from the surrounding air molecules otherwise the process would not be adiabatic.
The descending air does not cool surrounding molecules by drawing heat energy from them. Instead, the process of descending does work with gravity and warms the falling molecules up to the temperature of the surrounding molecules without addition or removal of heat energy from or to the surrounding molecules.
That heat energy can only be drawn from the store of gravitational potential energy carried by the molecule in addition to its kinetic energy.
Stephen 6:38pm: “I was simply agreeing that the energy did stay in the atmosphere, not asserting that it radiated to space.
Huh? No. Stephen confused the energy “it” with the adiabatic parcel “it”.
Trick clip by Stephen 4:17pm: ” energy…did NOT radiate to space”
Stephen 5:20pm: “Yes it did”.
So Stephen confuses using “it” for energy and adiabatic parcel at same time and argues most logically best he can by “Sheesh”.
6:38pm: “…it reduced the rate of cooling of the surface beneath…”
As far as I can tell, Stephen means by “it” the energy in the adiabatic 1st parcel for which as I have pointed out previously in the thread radiating to space is not possible as no energy of any kind crosses the adiabatic parcel border, the energy returns to the surface. Thus the adiabatic parcel cannot cool surface Tmean by radiating energy to space Stephen. At first cycle or any cycle ever since. This process is not responsible for the 33K as demonstrated by the top post agreeing with radiosonde test.
Need foundational cites supporting or arguing against top post picture Stephen not simply made up prose and no more confusing “it” use please.
******
Stephen 6:56pm: I count 8 uses of the word “it”. Make progress by reducing “it” count Stephen. Tell us what you really mean, back your assertion with cites. This will take some research, a library is nearby.
“You could see the point if you were not emotionally biased against it.”
I couldn’t have said it better! 😀
********************
I started to write more, but the permutations become endless …
* does the surface have a uniform temperature, or are we dealing with rotation & day/night?
* are we only dealing with a transparent atmosphere, or will we eventually include GHGs?
* does the planet start with the atmosphere or do we add it later? when we add it is it warmer, cooler, or the same as the planet?
* …
Your discussions are generally not specific enough to explain what would be happening at various times.
But I CAN tell you that with a totally transparent atmosphere, the “mass” cannot interact — either directly or indirectly — with any radiation from anywhere.
Tim said:
“does the surface have a uniform temperature, or are we dealing with rotation & day/night?”
A useful point to raise.
Rotation jumbles up the system response to incoming solar radiation but simplification by removing rotation is helpful.
If the Earth were not rotating there would be surface heating and atmospheric ascent ascent on the day side with surface cooling and atmospheric descent on the night side.
Energy taken up on the day side and converted to gravitational potential energy would descend on the night side and be reconverted to warm air above the surface.
That warm air returning to the surface on the night side would reduce net night side radiative cooling below what it would have been in the absence of the atmosphere.
By reducing night side cooling the average temperature of the entire sphere would rise 33C above S-B in the case of Earth.
Tim said:
“with a totally transparent atmosphere, the “mass” cannot interact — either directly or indirectly — with any radiation from anywhere”
Then please account for conduction and convection.
Radiation comes in, some is diverted to conduction within mass and radiation goes out.
How is that not the interaction of mass with radiation ?
The truth is that that interaction between radiation and mass slows down the transmission of solar radiation through the Earth system and that slowing down causes the surface temperatutre enhancement above the S-B prediction. No GhGs needed.
Trick said:
“Thus the adiabatic parcel cannot cool surface Tmean by radiating energy to space Stephen. ”
Agreed, it cannot. If you had absorbed my earlier comments there would have been no confusion. I have consistently said that the net effect of convective overturning is to warm a surface after the first convective cycle completes and the effect on the surface temperature is zero during the first cycle.
Mind you, Tim thinks it can cool the surface below S-B but I have said why that is impossible.
Anyway, it does warm the surface at the end of the first convective cycle when returning kinetic energy on the descent slows down surface cooling for as long as the atmosphere remains suspended off the surface by convection. Effectively forever.
Since the conductively warmed atmosphere slows down surface cooling the surface temperature must rise by 33C to regain radiation balance with space at the same time as it supplies the energy needed to maintain atmospheric height.. No GHGs needed.
The fact is that for Earth the surface energy cost of keeping the weight of the atmosphere suspended off the surface is equivalent to a surface temperature enhancement of 33C.
Stephen 10:13pm: “Then please account for conduction and convection.”
Ok.
In an extremely thin optical depth atmosphere on earth, the dirt/water surface would equilibrate with atmosphere in contact in account for conduction and convection and what little radiative transfer would exist. The lapse rate would start from just above Tmean=255K in this epoch and reduce with z height by 1st law (conservation of enthalpy) and Planck radiation distribution. This is demonstrated by the top post picture being in agreement with radiosonde data.
IR active gas (GHG) affects optical depth and can make atmosphere thick as Tmean=288K demonstrates by 1st law & Planck distribution.
There really is no use in discussing a totally transparent atmosphere as that is akin to discussing the results of dividing by zero.
“The truth is that that interaction between radiation and mass..surface temperatutre enhancement above the S-B prediction..”
All radiation comes from mass by Planck distribution which tells us surface temperature is never above S-B prediction; surface Tmean is in accord with Planck distribution. Always and everywhere. No exceptions have ever been found in nature unless the object had negative radii or non-negligible diffraction. Top post analytical picture is confirmation of this as agrees with radiosonde test.
0 use of “it”.
******
10:25pm: ”…net effect of convective overturning is to warm a surface…”
No. Stephen still does not ever get ALL equilibrium adiabatic cycles including the 1st one cannot warm the surface as they are adiabatic. NO energy transfer – at all by any means. This is confirmed by the top post picture registering the same as radiosonde test.
“Mind you, Tim thinks it can cool the surface below S-B but I have said why that is impossible.”
It? Where does Tim write that?
“Since the conductively warmed atmosphere slows down surface cooling the surface temperature must rise by 33C…”
No Stephen. This is meaningless as conduction/convection are in equilibrium, become adiabatic processes – no net energy transfer over a decade or eon – for surface Tmean as shown by the top post picture in agreement with radiosonde test. The top post picture derived from radiative transfer alone means the 33K is entirely from the thick optical depth of earth atmosphere. In accord with satellite and thermometer field measurement.
Will says: “What wierd thinking! No “heat energy” transfered to a lower temperature atmosphere, is ever returned to the higher temperature surface by any means whatsoever.”
Certainly on a planet with a uniform surface temperature you would be correct. On the other hand, the energy could be taken from times and places where the land is warm (eg during the day) and returned at times an places where the land is cool (eg at night). It is such temperature differences that drive convection in the first place — a uniformly warm planet would not have convection.
I have no problems with what you write about evaporation.
I am not a huge fan of how you use “heat” but I can live with it.
“For each WV molecule having only cross sectional area and quite sparce in the atmosphere, each WV molecule is free to radiate equally into a whole very low radiance hemisphere at all wavelengths from 14 to 200 microns.”
At this point I would say if each molecule has only its own space, it has no knowledge of what is happening, and can’t “decide” not to radiate in any particular direction.
Furthermore, even if you want to say it somehow knows whether the molecule that might receive the energy is higher energy (warmer) or lower energy (colder), any collection of molecules has both high and low energy molecules. Some molecules of water in a gas at 280 K are higher energy than some molecules of water at 300 K (Surely you accept the idea of molecules and the Maxwell-Boltzmann distribution) . Does that mean that in your worldview, those exceptionally high energy water molecules in the cool gas can radiate to the exceptionally cool molecules in the warm gas? Or does that high energy molecule in the cool gas “know” that it is surrounded by lower energy molecules and that the low energy molecule that it wants to radiate to is surrounded by higher energy molecules and thus choose not to go there based on a knowledge of hte entire MB distribution of both gases???
****************************************************
The reason the net flow of energy is from warm to cool is the same reason the net flow of money is from gamblers to casinos — probability. An individual gambler might win money on a particular night, but in the end, the house always wins because the odds are in its favor. If I bet “heads” every time on a coin that comes up heads 51% of the time, I will eventually come out ahead. If I only perform the experiment 100 times, there is a good chance I will come out behind. If I perform the experiment 1,000,000 times, there is a hardly any chance I will come out behind. If I perform the experiment 10^23 times, there is a basically no chance I will come out behind.
Photons flaying around between a mole of warm water vapor and a mole of cooler water vapor is such an experiment. The odds are always in favor of energy moving from a molecule in the warm gas to a molecule in the cool gas. If look only at a single molecule in the warm gas and a single molecule in the cool gas, sometimes the energy DOES go “the wrong way (just like the coin that was altered to come up heads 51% of the time still comes up tails some times). But given the astronomically large numbers involved, the net result is always seen to favor the more probably outcome.
Stephen, this sentence is perhaps the crux of your misunderstanding:
“The truth is that that interaction between radiation and mass slows down the transmission of solar radiation through the Earth system and that slowing down causes the surface temperatutre enhancement above the S-B prediction. No GhGs needed.”
GHGs are a large, important component of “that interaction between radiation and mass” (other major parts being aerosols and clouds). An atmosphere of pure N2 (or O2 or Ar) would have almost no interaction with the outbound thermal IR and would have almost no impact on surface temperatures (no matter how thick that N2 atmosphere). Yes, it would reduce the variations between night and day, but it would NOT raise the temperature above the SB predictions. With a transparent atmosphere that would so clearly violate conservation of energy.
By simply sayin the words “interaction between radiation and mass” you are agreeing that the atmosphere is NOT transparent!
So, yes, if there is an interaction between the outgoing thermal IR and the atmosphere, then the atmosphere will cause the sort of warming you describe. But without those interactions with GHGs (or aerosols or clouds), no amount of atmosphere will get you above the simple SB limits.
gallopingcamel says, September 15, 2014 at 3:17 pm:
“I was hoping you would be impressed by the excellent “fit” achieved by the R&C model for the HASI probe data (Titan). In my opinion that is a tremendous achievement.”
I am impressed. Don’t worry. However, I hope it’s still allowed to raise objections …
Stephen Wilde says, September 15, 2014 at 11:17 am:
““An adiabatic process is a process that occurs without the transfer of heat or matter between a system and its surroundings.”
http://en.wikipedia.org/wiki/Adiabatic_process
Thus adiabatic descent does NOT involve transfer of heat or energy from the surrounding gases.”
Stephen, at least try to understand the distinction between heat and energy. Heat is ALWAYS energy. Energy is NOT always heat.
In an adiabatic process, energy is per definition NOT transferred to or from the system in question as HEAT [Q], that is, warming and cooling of that system is NOT accomplished by a transfer across the system boundary of energy AS HEAT. The warming/cooling of the system is rather accomplished by a transfer across the system boundary of energy AS WORK [W].
Both HEAT and WORK are transfers of energy to/from a thermodynamic system. They are both capable of changing the internal energy [U] (and hence, the temperature) of that system. ‘Adiabatic’ mean NO HEAT transferred. It doesn’t mean no ENERGY transferred. The energy is transferred in the form of WORK, Stephen. WORK. Expansion and compression. This is the whole point with the adiabatic process.
“The surrounding gases do NOT cool or become less energetic as heat is created within the descending air.”
Yes, they do. And heat is NOT created within an air parcel. HEAT is not contained within a thermodynamic system!!! It is transferred to/from it. By virtue of the temperature difference between it and its surroundings.
“The heating in adiabatic descent comes from WITHIN the descending parcel via conversion of gravitational potential energy to kinetic energy. The total amount of energy in each molecule remains the same but it changes form with uplift and descent.”
No, it doesn’t. The warming and cooling comes specifically from the compression (on descent) and expansion (on ascent) of the gas. This is what the adiabatic process is all about. No expansion of the air, no cooling. It doesn’t warm simply from being moved lower. No compression of the air, no warming. It doesn’t cool simply from being mover higher. The air parcel needs to contract and expand to have its temperature changed in an adiabatic process. When the air expands, it does WORK [W] on its surroundings, losing energy. Energy is transferred from the expanding parcel to the surrounding air which it ‘pushes away’. The opposite happens on descent.
“It is then returned as heat on the descent and ADDS to the heat generated by the flow of solar radiation passing through the system. That is why it raises the temperature above S-B by 33C.”
NO! NO! NO!!! Heat can not be transferred from a cool atmosphere to a warm surface, Stephen. That’s a direct violation of the 2nd Law of Thermodynamics. Heat only moves spontaneously from hot to cold. You MUST read up on the thermodynamic concept of ‘heat’. I’m afraid you have a huge hole in your understanding here … And instead of learning, you make up your own reality.
“This is what the adiabatic process is all about. No expansion of the air, no cooling. It doesn’t warm simply from being moved lower. No compression of the air, no warming. It doesn’t cool simply from being mover higher.”
Late in the evening. Should be:
“This is what the adiabatic process is all about. No expansion of the air, no cooling. It doesn’t cool simply from being mover higher. No compression of the air, no warming. It doesn’t warm simply from being moved lower.”
A process which takes place is sublimation of ice.
This involves a huge energy flow without a change in heat, no temperature change.
However this can be viewed using various terminology, meaning of words varies
http://chemwiki.ucdavis.edu/Physical_Chemistry/Thermodynamics/State_Functions/Enthalpy/Heat_of_Sublimation
Kristian 1:19am: There is no need to invoke the confusing “heat” term to explain an adiabatic process in the context of an atmosphere parcel, you will only confuse things further. Try this simpler one:
To determine the DALR, consider the rate of decrease of temperature with height of a parcel of air that ascends (or descends) in such a way that its pressure is always that of the surrounding air. During this ascent the parcel does not exchange any energy with its surroundings by virtue of a temperature difference between them (hence “adiabatic”), and any water vapor the parcel may contain is not transformed into liquid or ice (hence “dry”).
Wasn’t that easier, less confusing than your use of “heat” term? It should be on word count alone. From this should be obvious enthalpy is conserved in the process as the parcel cannot exchange energy with the surface or with anything at any height. Stephen continuously misses this science when he writes descending parcels can warm the surface 33C. Make definitions simple, less confusing enable Stephen et. al. to grasp the correct concept. Eventually.
Demonstration of this science is in the top post picture analysis = testing in real atmosphere by radiosonde.
******
Tim C. 2:03am: Good discussion of enthalpy in your link. Thanks. Educational. For the atmosphere parcel enthalpy is conserved quantity equal to h = internal energy + p*V = PE + KE + p*V.
Note the h was chosen for parcel enthalpy in olden times from the word “heat”. That was olden times, Now it is modern times, shortened to just h.
Tim Folkerts says: September 16, 2014 at 9:10 pm
“But I CAN tell you that with a totally transparent atmosphere, the “mass” cannot interact — either directly or indirectly — with any radiation from anywhere.”
Please give one physical example of ‘totally transparent atmosphere”? Your imagination astounds!
Every physical mass interacts with electromagnetic radiation (flux) at every frequency. Not even space in the Solar system is transparent.
Stephen Wilde says: September 16, 2014 at 10:08 pm
“Energy taken up on the day side and converted to gravitational potential energy would descend on the night side and be reconverted to warm air above the surface.”
Please demonstrate how heat, sensible or latent, can ever be converted to mass positional gravitational energy? A thermos of hot coffee will retain its sensible heat all the way to 15
kilometers while gaining much potential energy.
“That warm air returning to the surface on the night side would reduce net night side radiative cooling below what it would have been in the absence of the atmosphere.”
The WV if any, in the lower atmosphere, with its specular radiance at that temperature, does indeed reduce the EMR flux from a higher temperature surface. This is never a thermodynamic process, it is an electromagnetic state controling the amount if EMR flux, which is never heat energy.
“By reducing night side cooling the average temperature of the entire sphere would rise 33C above S-B in the case of Earth.”
Only in your fantasy! There is no S-B case with a electromagnetically disbursuve atmosphere in a gravitational field. Any predicted temperatures are only fraud. Even Planck’s integral is not applicable anywhere near this planet The specular emissivity of every mass is changing and unknown! Near this Earth not even Navier-Stokes partial differential equations can be used. Not enough is known for even a guess.
Will says: “Please give one physical example of ‘totally transparent atmosphere”?”
First of all, it is not my model. I am merely commenting on Stephen’s model. He claims atmosphere can warm a surface above the limits set by the SB equation, which violates several major laws of thermodynamics.
********************************
But beyond that, it is *absolutely* *common* to make idealizations when discussing problems. Every engineering student at some time does a problem with a frictionless surface or a massless pulley or a rigid rod or a battery with no internal resistance or an incompressible fluid or ….
Rather than being pointless, these are important steps along the way toward understanding. If you don’t have the ability to recognize the importance of approximations and idealizations … well then your LACK of imagination astounds!
Trick says: September 16, 2014 at 10:56 pm
“All radiation comes from mass by Planck distribution which tells us surface temperature is never above S-B prediction; surface Tmean is in accord with Planck distribution. Always and everywhere. No exceptions have ever been found in nature unless the object had negative radii or non-negligible diffraction. Top post analytical picture is confirmation of this as agrees with radiosonde test.”
There is no S-B case with a electromagnetically disbursive atmosphere in a gravitational field. Any predicted temperatures are only fraud. Even Planck’s integral is not applicable anywhere near this planet The specular emissivity of every mass is changing and unknown! Near this Earth not even Navier-Stokes partial differential equations can be used. Not enough is known for even a guess.
Any spherical concave mirror does indeed have negative curvature for EMR. Quite handy for some concept of what is! Totally disconected from fantasy computer models.
“0 use of “it”.”
My non use of “it” trumps your use of nonsense!
Your use of diffraction only comes from the Max Planck Institute “theory” on powder diffrection, and is limited to x-rays and high energy charged particles. Never anything written by friendly Max. To the rest of the world, diffraction of sunlight, by partially condensed drizzle drops, results in observable rainbows! Please flush your crap before returning to your seat.
@Kristian,
“I am impressed. Don’t worry. However, I hope it’s still allowed to raise objections …”
Our understanding of planetary atmospheres is still pathetic so there is plenty of room for objections. Objections should help us find ways to improve existing theories.
You point out that on Earth the tropopause transition occurs over a broad range of pressures (0.1 to 0.2 bars). On Titan the range is even greater (0.05 to 0.50 bars). R&C have an explanation based on stability criteria. They see the troposphere as unstable compared to the stratosphere. The tropopause is the transition region.
Nikolov & Zeller suggested that pressure is a more important variable than gas composition. From my perspective R&C have given this idea a stronger mathematical basis. We already know that cloud top heights are primarily determined by total gas pressure and now it seems that the tropopuase is defined in the same way.
So the question at the top of this post is a good one. Does CO2 have a significant effect?
Tim Folkerts says: September 16, 2014 at 11:39 pm
(Will says: “What wierd thinking! No “heat energy” transfered to a lower temperature atmosphere, is ever returned to the higher temperature surface by any means whatsoever.”)
“Certainly on a planet with a uniform surface temperature you would be correct. On the other hand, the energy could be taken from times and places where the land is warm (eg during the day) and returned at times an places where the land is cool (eg at night). It is such temperature differences that drive convection in the first place — a uniformly warm planet would not have convection.”
Hell! Even a uniform temperature surface must have convection due to the low density WV created always by Solar EMR.
“I have no problems with what you write about evaporation.
I am not a huge fan of how you use “heat” but I can live with it.”
I will try to use “heat energy”, to distinguish that from Newtonian kinetic, Mass with vector motion, versus heat energy with no vector momentum!
(“For each WV molecule having only cross sectional area and quite sparce in the atmosphere, each WV molecule is free to radiate equally into a whole very low radiance hemisphere at all wavelengths from 14 to 200 microns.”)
“At this point I would say if each molecule has only its own space, it has no knowledge of what is happening, and can’t “decide” not to radiate in any particular direction.”
Each molecule exists in a thermal exists in a thermal environment at its own temperature. For electromagnetic radiative flux That is decided externially by opposing radiances at that frequency.
Spontaneous EMR flux is only in the direction of lower radiance. and the amount of flux limited by the difference in opposing radiance at each frequency.
”Furthermore, even if you want to say it somehow knows whether the molecule that might receive the energy is higher energy (warmer) or lower energy (colder), any collection of molecules has both high and low energy molecules. Some molecules of water in a gas at 280 K are higher energy than some molecules of water at 300 K (Surely you accept the idea of molecules and the Maxwell-Boltzmann distribution) . ”
No knowlege is required Go learn your own nonsensical statistical mechanics, At 300 Kelvin a 5 kelvins difference between surfaces nearly black, already have a six sigma difference in possible energy transfer. At what point will you agree that all thermal EMR “flux” is unidirectional?
“Does that mean that in your worldview, those exceptionally high energy water molecules in the cool gas can radiate to the exceptionally cool molecules in the warm gas? Or does that high energy molecule in the cool gas “know” that it is surrounded by lower energy molecules and that the low energy molecule that it wants to radiate to is surrounded by higher energy molecules and thus choose not to go there based on a knowledge of hte entire MB distribution of both gases???”
Tim I have no worldview! I only have my measurements that I try to convey “meaning” of measurement “with the least possible confusion”.
.
Tim Folkerts says: September 17, 2014 at 12:12 am
Stephen, this sentence is perhaps the crux of your misunderstanding:
“The truth is that that interaction between radiation and mass slows down the transmission of solar radiation through the Earth system and that slowing down causes the surface temperatutre enhancement above the S-B prediction. No GhGs needed.”
“GHGs are a large, important component of “that interaction between radiation and mass” (other major parts being aerosols and clouds). An atmosphere of pure N2 (or O2 or Ar) would have almost no interaction with the outbound thermal IR and would have almost no impact on surface temperatures (no matter how thick that N2 atmosphere). Yes, it would reduce the variations between night and day, but it would NOT raise the temperature above the SB predictions. With a transparent atmosphere that would so clearly violate conservation of energy.”
What conservation of energy is required for an atmosphere that is free to dispatch any amount of entropy to space via adjustable EMR?
“By simply sayin the words “interaction between radiation and mass” you are agreeing that the atmosphere is NOT transparent!”
It is not transparant. It is at radiative equilibrium. As such, atmosphere attenuates no thermal EMR flux from a higher temperature below.
Kristian says: September 17, 2014 at 1:19 am
Stephen Wilde says, September 15, 2014 at 11:17 am:
““An adiabatic process is a process that occurs without the transfer of heat or matter between a system and its surroundings.”
http://en.wikipedia.org/wiki/Adiabatic_process
Thus adiabatic descent does NOT involve transfer of heat or energy from the surrounding gases.”
Stephen, at least try to understand the distinction between heat and energy. Heat is ALWAYS energy. Energy is NOT always heat.
In an adiabatic process, energy is per definition NOT transferred to or from the system in question as HEAT [Q], that is, warming and cooling of that system is NOT accomplished by a transfer across the system boundary of energy AS HEAT. The warming/cooling of the system is rather accomplished by a transfer across the system boundary of energy AS WORK [W].
Both HEAT and WORK are transfers of energy to/from a thermodynamic system. They are both capable of changing the internal energy [U] (and hence, the temperature) of that system. ‘Adiabatic’ mean NO HEAT transferred. It doesn’t mean no ENERGY transferred. The energy is transferred in the form of WORK, Stephen. WORK. Expansion and compression. This is the whole point with the adiabatic process.
“The surrounding gases do NOT cool or become less energetic as heat is created within the descending air.”
Yes, they do. And heat is NOT created within an air parcel. HEAT is not contained within a thermodynamic system!!! It is transferred to/from it. By virtue of the temperature difference between it and its surroundings.
“The heating in adiabatic descent comes from WITHIN the descending parcel via conversion of gravitational potential energy to kinetic energy. The total amount of energy in each molecule remains the same but it changes form with uplift and descent.”
“The surrounding gases do NOT cool or become less energetic as heat is created within the descending air.”
Yes, they do. And heat is NOT created within an air parcel. HEAT is not contained within a thermodynamic system!!! It is transferred to/from it. By virtue of the temperature difference between it and its surroundings.
“The heating in adiabatic descent comes from WITHIN the descending parcel via conversion of gravitational potential energy to kinetic energy. The total amount of energy in each molecule remains the same but it changes form with uplift and descent.”
No, it doesn’t. The warming and cooling comes specifically from the compression (on descent) and expansion (on ascent) of the gas. This is what the adiabatic process is all about. No expansion of the air, no cooling. It doesn’t warm simply from being moved lower. No compression of the air, no warming. It doesn’t cool simply from being mover higher. The air parcel needs to contract and expand to have its temperature changed in an adiabatic process. When the air expands, it does WORK [W] on its surroundings, losing energy. Energy is transferred from the expanding parcel to the surrounding air which it ‘pushes away’. The opposite happens on descent.
“It is then returned as heat on the descent and ADDS to the heat generated by the flow of solar radiation passing through the system. That is why it raises the temperature above S-B by 33C.”
NO! NO! NO!!! Heat can not be transferred from a cool atmosphere to a warm surface, Stephen. That’s a direct violation of the 2nd Law of Thermodynamics. Heat only moves spontaneously from hot to cold. You MUST read up on the thermodynamic concept of ‘heat’. I’m afraid you have a huge hole in your understanding here … And instead of learning, you make up your own reality.
—————————————————————————————————————————-
Very nicely done Kristan!
tchannon says: September 17, 2014 at 2:03 am
“A process which takes place is sublimation of ice.
This involves a huge energy flow without a change in heat, no temperature change.
However this can be viewed using various terminology, meaning of words varies
http://chemwiki.ucdavis.edu/Physical_Chemistry/Thermodynamics/State_Functions/Enthalpy/Heat_of_Sublimation”
That sublimation is very endothermic 3000 J/g for the chemical conversion of solid H20 to WV.
This is called Latent heat, not sensible heat. Carefully done, it is a reversable thermodynamic process with all Joules recovered as sensible heat as that WV reconverts back to ice.
That latent heat is completely devoid of any change to potential gravitational energy, based on its position in space. In this atmosphere it is storage for sensible heat. More importantly it is the energy source for EMR entropy flux to space, from the atmosphere, not any surface. This has not anything to do with the very precise S-B equation for “maximum” spontaneous thermal radiative power transfer between surfaces at different temperatures.
Tim Folkerts says: September 17, 2014 at 4:32 am
(Will says: “Please give one physical example of ‘totally transparent atmosphere”?”)
“First of all, it is not my model. I am merely commenting on Stephen’s model. He claims atmosphere can warm a surface above the limits set by the SB equation, which violates several major laws of thermodynamics.”
I.E. you have not even a conceptual example! Discard all learning, substitute fantasy!
Thermostatic state with gravitational attraction of N2 and O2, demonstrate your nonsense.
No Tim, no Stephen’s model, Your fantasy with your intent to confuse! Any body can be at any temperature without regard to any S-B equation. The carefully crafted and very precise S-B equation for “maximum” spontaneous thermal radiative power transfer between surfaces at different temperatures. That S-B equation has no temperature limits on or near this planet. You have no concept of how internal energy increases or decreases in this Earth. You have nothing but arrogant fantasy, no physics whatsoever! This is your fist (Pointman). Poor students!
The work done in adiabatic uplift and descent is done against gravity and does not involve any transfer of heat to or from adjoining molecules.
The work done between the moving parcel and the gravitational field not between the moving parcel and adjoining molecules.There is movement of adjoining molecules caused by the expansion or contraction but the thermal effect on the adjoining molecules is zero for a truly adiabatic scenario.
The work done with or against the gravitational field does not create new energy nor destroy existing energy. Instead, it converts kinetic to potential and back again by causing expansion or compression.
As E M Smith correctly said:
“Descending air is turning potential energy of height (gravity) into thermal (compression). Rising air turns thermal into potential doing work against gravity. There is energy exchange going on, just not heat flows (themal to thermal).”
Kristian said:
“Stephen, at least try to understand the distinction between heat and energy. Heat is ALWAYS energy. Energy is NOT always heat.”
That is what I have been telling you.
Adiabatic uplift turns heat energy to non heat energy (gravitational potential energy).
Adiabatic descent turns non heat energy to heat energy.
You need to think through the practical implications of that though I’ve set it out for you clearly enough several times already.
Kristian said:
“NO! NO! NO!!! Heat can not be transferred from a cool atmosphere to a warm surface, Stephen”
I’ve never said it did.
Warm air above a cool surface will slow the rate of surface cooling which is all I have claimed.
Will said:
“By simply sayin the words “interaction between radiation and mass” you are agreeing that the atmosphere is NOT transparent!”
That is a non sequitur because a completely transparent atmosphere will still acquire energy from an irradiated surface by conduction and conduction is merely radiation that is temporarily stored within mass.
Storage of previously radiative energy within mass is an interaction between radiation and mass.
It is the length of time of that storage which causes the temperature rise and that length of storage time is extended substantially by the convective cycle.
Will Janoschka says, September 17, 2014 at 3:58 am:
“Please demonstrate how heat, sensible or latent, can ever be converted to mass positional gravitational energy? A thermos of hot coffee will retain its sensible heat all the way to 15
kilometers while gaining much potential energy.”
Exactly. A parcel of air does not cool simply from rising. It cools because it expands while rising. That is what adiabatic cooling is all about. Expansion of a volume of air involves work [W] being done by the air on the surrounding air in pushing it to the side.
Stephen doesn’t appear to understand this at all.
To help resolve some of the apparent confusion over the diabatic / adiabatic concepts as applied to an atmosphere, please note the following.
i) The initial warming of a surface is diabatic due to energy from sunlight being added.
ii) Such warming can never be even so areas that are warmed more than others conduct more energy to the air above.
iii) Those favoured more warm parcels of air become less dense and lighter than adjoining parcels and start to rise.
iv) That will happen even with no GHGs in an atmosphere that is completely transparent to radiation.
v) Uplift is in the form of bubbles rather than a continuous stream because the rising parcel takes heat away from the surface which then cools enough for the density differential with adjoining parcels to disappear. If uneven insolation continues to favour the same surface location then the process will repeat with another bubble rising subsequently.
vi) Being in bubble form there is a point at which the parcel detaches from the surface. At that point all further uplift becomes adiabatic with no more energy needing to be added to enable indefinite uplift to occur.
vii) In practice the uplift eventually stops when the parcel reaches a temperature inversion (the tropopause) because the warmer temperature at the inversion layer equalises the densities inside and outside the parcel whereupon uplift ceases.
vii) The reason one can have indefinite adiabatic uplift is because the decline in pressure with height and subsequent expansion of the parcel preserves the density differential between the parcel and its surroundings. The surroundings become less dense at the same rate as the parcel becomes less dense so the density differential at the surface that began the process is preserved, potentially right up to the top of the atmosphere.
viii) At the top of the atmosphere or at the tropopause the parcel stops rising but more bubbles keep coming up from below and the parcel is pushed to one side.
ix) Having been pushed to one side and having cooled and become denser than its surroundings the parcel then starts to descend in a location remote from the region of uplift. Hence descending high pressure cells interspersed with rising low pressure cells in meteorology.
x) The whole process then reverses with the parcel becoming warmer throughout descent without addition of any new energy as heat.
xi) Work is being done throughout uplift and descent but the work is being done against and with gravity and so has zero thermal effects on the surrounding air molecules. No work is done against other air molecules if a parcel ascends into less dense air. Similarly no work is done against other air molecules when a parcel is descending into more dense air because the work is being done with gravity.
xii) Eventually the parcel arrives at the surface and once it touches the surface the adiabatic process ends and diabatic processes take over once more.
xiii) By that ever changing convective process, surface heat energy can be ‘rationed’ as necessary via variable convective uplift and descent to ensure that the surface temperature never rises more than 33C above S-B for Earth’s particular atmospheric mass. The store of gravitational potential energy in the atmosphere is a variable heat sink or reservoir which can be called upon whenever any change in the composition of the atmosphere tries to destabilise the system. It works with or without GHGs because it relies on conduction and not radiation.
Stephen Wilde says: September 17, 2014 at 9:33 am
(Kristian said: “NO! NO! NO!!! Heat can not be transferred from a cool atmosphere to a warm surface, Stephen”)
“I’ve never said it did.Warm air above a cool surface will slow the rate of surface cooling which is all I have claimed.”
Air with some WV at the same temperature as the surface will prevent any radiative exitance from the surface because of its opposing radiance. This is an electromagnetic property or state. This has nothing to do with any thermodynamic process. At the same time that air with WV replaces surface exitance with a greater exitance because of increasing radiative solid angle to space. This increase continues all the way to the tropopause, measured. That opposing radiance does nothing to prevent convection and latent heat tranfer to the upper troposphere where it is merrily dispatched to space, along with all other entropy, via EMR flux. None of this heat energy, sensible or latent, ever returns to the higher temperture surface by any means whatsoever. This is not a steam engine, please try to get a clue as to how a planet the size of Earth may possibly work.
This Earth can, if it wishes, put no WV to the atmosphere and surface temperature must increase untill the oceans boil for atmospheric WV to cool off. 373 Kelvin. Or this same Earth, if it wishes, can put so much atmospheric WV via ice sublimation, so all oceans are frozen solid. 240 Kelvin. Will someone please try to figure what is? That S-B nonsense is but deliberate fraud.
“no work is done against other air molecules when a parcel is descending into more dense air because the work is being done with gravity.”
To clarify that point the reason no work needs to be done against surrounding molecules in the descent is that gravity compresses the descending parcel as it descends which makes any work against other molecules unnecessary.
Work against other molecules is only needed if a less dense parcel tries to retain its lower density whilst pushing denser parcels aside. Gravitational compression removes the need for that.
Stephen Wilde says, September 17, 2014 at 9:33 am:
“Kristian said:
“NO! NO! NO!!! Heat can not be transferred from a cool atmosphere to a warm surface, Stephen”
I’ve never said it did.
Warm air above a cool surface will slow the rate of surface cooling which is all I have claimed.”
Then say exactly that. Not this: “It is then returned as heat on the descent and ADDS to the heat generated by the flow of solar radiation passing through the system. That is why it raises the temperature above S-B by 33C.” You sound totally confused about the laws of thermodynamics when stating your case like this. Nothing is ‘returned as heat on descent’, Stephen. And certainly not adding to any solar heat. Stating it like this will make people believe that you think ‘heat’ can be brought spontaneously from a cooler place and down to a warmer place, a direct violation of the 2nd Law.
Stephan
To help you get out of your mind block on this topic I gave you a worked example.
Loss of internal energy for a mole of air at STP rising to a height of 1000m is 203J
Work done against surroundings is 203J (use of barometric equations)
So not one Joule of lost internal energy was turned into gravitational potential energy!
And yet the mole of air is now 1000m from the ground with this mass of air having apparently acquired 284J
I can see your problem in accepting these undoubted facts!
The mole of gas at ground level would occupy 22.4 litres but at 1000m would have expanded considerably.
(I can work out the exact volume if that helps)
Now think of it this way!
The air parcel RISES but an almost exactly similar parcel FALLS to replace the void left.
The falling parcel is to all intents exactly the same as the rising parcel
Rising one gaining PE and the falling one losing PE in exactly equal steps
The parcel at 1000m would lose exactly 243J of PE on returning to ground level.
The parcel would be compressed on the way down by its surroundings and thus would gain exactly 203J and its temperature would have risen to 273K and would occupy a volume of 22.4l.
The first law of thermodynamics has been satisfied in all respects.
Now back to the main point of this thread
What changes does a radiative gas fraction make to this situation?
For similar volumes of gases in the lower troposphere the effects are largely self cancelling
As the parcel moves higher to thinner air there will be a gradual greater loss through upward emission than absorption and an increasing heat transfer to space.
So our parcel would lose internal energy by this mechanism and start to descend as it is slightly denser than its surroundings
And when is warm air situated above a cool surface other than in local/regional settings? This never happens globally. What do you mean by this, Stephen? If warm air were lying on top of a cool surface, then that warm air would indeed increase the HEATING of that surface, by transferring energy to it as HEAT. It wouldn’t reduce its COOLING.
“Air with some WV at the same temperature as the surface will prevent any radiative exitance from the surface because of its opposing radiance. This is an electromagnetic property or state. This has nothing to do with any thermodynamic process”
Thermodynamic processes cause non radiative air to be warmer than the cold of space in the first place.
As I keep telling you, the radiative fluxes within an atmosphere are the consequence of non radiative thermodynamics and not the cause.
“And when is warm air situated above a cool surface other than in local/regional settings?”
On average for 50% of the globe.
Ever heard of night or winter ?
The mass of conductively warmed air brought down adiabatically from higher levels prevents a cooling surface from cooling as much as it would without an atmosphere. A breeze on a clear night can stop a frost forming or even raise a frozen surface above freezing by reducing net radiative loss to space.
Reducing the extent to which the night / winter side can cool has the effect of raising Earth’s average surface temperature by 33C.
@gallopingcamel
Peter, I have a question. You used a multi layer FEA model to replicate Diviner empirical results.
Some time back I build and ran an empirical experiment involving the depth of SW absorption and equilibrium temperature in transparent materials –


Here is what is happening during the rise to equilibrium –

Keeping SW absorption and IR emissivity identical and only altering depth of SW absorption a dramatic 20C temperature differential between the test blocks develops in 3 hours of solar exposure.
Would you be able to replicate this “selective surface” effect using the same multi-layer FEA approach?
“If warm air were lying on top of a cool surface, then that warm air would indeed increase the HEATING of that surface, by transferring energy to it as HEAT. It wouldn’t reduce its COOLING.”
A surface beneath a clear sky radiates readily to space and cools the air above it in the process. It draws energy from the air by conduction.
The energy drawn from the air results in the surface not cooling as fast as if there were no atmosphere.
If there is wind constantly replacing the energy drawn from the air by the radiating surface then the temperature fall for the surface will be even less.
Bryan,
Joules are merely a measure of the amount of work done with or against gravity in achieving the change in height.
Joules are not a measure of internal energy.
http://dictionary.reference.com/browse/joule
Stephen
Are you taking the piss?
Will 4:53am: “There is no S-B case with a electromagnetically disbursive atmosphere in a gravitational field.”
Will is refuted by the top post picture analysis = test performed by radiosonde. Planck distribution and S-B therefore work as observed in an atmosphere.
6:17am: “What conservation of energy is required for an atmosphere that is free to dispatch any amount of entropy to space via adjustable EMR?”
In an atmosphere parcel, enthalpy is the conserved quantity by 1st law. Energy in – Energy out of parcel = change in internal energy of parcel. Entropy always increases in natural processes. Rather simple Will.
******
Stephen 10:50am: ” iv) That will happen even with no GHGs in an atmosphere that is completely transparent to radiation.”
More physical: That will happen even with an optically thin atmosphere.
“It works with or without GHGs because it relies on conduction and not radiation.”
It?? The convective process would work as Stephen generally writes on earth with extremely optically thin atmosphere at surface Tmedian a little above 255K and with optically thick natural atmosphere as on earth at surface Tmedian around 288K. By 1st law and Planck distribution as demonstrated by top post analysis = radiosonde data.
11:48am: ”Joules are not a measure of internal energy.”
I can assure Stephen that joules are a unit of internal energy. Whatever gave Stephen this idea? Sheesh.
******
Konrad 11:27am: “Would you be able to replicate this “selective surface” effect using the same multi-layer FEA approach?”
Judicious use of 1st law, Fourier conduction and Planck distribution will work to analyze Konrad’s experiment just like they do in the top post picture in the atmosphere analysis = radiosonde data. Why not perform the analysis=data workout for the experiment yourself Konrad?
Stephen Wilde says, September 17, 2014 at 11:26 am:
““And when is warm air situated above a cool surface other than in local/regional settings?”
On average for 50% of the globe.
Ever heard of night or winter ?”
This is just getting silly. What game are you playing here?
Is the air above the surface warmer than the surface as soon as the Sun sets, Stephen? Meaning, over 50% of the globe at any specific time? So there’s a full hemispheric inversion going on at all times, according to you?
Are typical high latitude winter conditions present over 50% of the globe at any point in time? And is the air warmer than the surface wherever and whenever such winter conditions prevail?
Bryan says, September 17, 2014 at 11:54 am:
“Stephen
Are you taking the piss?”
I think he is.
Kristian says: September 17, 2014 at 11:17 am
“And when is warm air situated above a cool surface other than in local/regional settings? This never happens globally. What do you mean by this, Stephen? If warm air were lying on top of a cool surface, then that warm air would indeed increase the HEATING of that surface, by transferring energy to it as HEAT. It wouldn’t reduce its COOLING.”
Happens over each tropical desert, that is why no generation of WV there.
Be careful of the word “heating”. This is a ClimAstrology weasel word. Increasing the sensible heat of that mass is what you mean, I think. I once thought that had a kitten, now I know the Cat has in earthling, ME.
Bryan,
I take the point about Joules but found this:
“In thermodynamics, the internal energy is one of the two cardinal state functions of the state variables of a thermodynamic system. It refers to energy contained within the system, while excluding the kinetic energy of motion of the system as a whole and the potential energy of the system as a whole due to external force fields. It keeps account of the gains and losses of energy of the system.”
http://en.wikipedia.org/wiki/Internal_energy
Since the force of gravity is an external force field it affects the potential energy of the system as a whole.
Movement of mass within the gravitational field appears to cause KE to switch to PE and vice versa.
All your calculation seems to show is that once the adiabatic convective cycle has completed the first loop there is no further change in internal energy content. I have said that myself.
However, until the first cycle of the loop closes there is an on going accumulation of potential energy caused by the first convective cycle.
During the initial development of convection the system is acquiring increasing internal energy in the form of that additional potential energy being created within the atmosphere as result of work being done against gravity.
It is that increased internal energy which causes the 33C temperature rise as soon as the adiabatic loop first closes.
Stephen Wilde says: September 17, 2014 at 11:20 am
(WJ “Air with some WV at the same temperature as the surface will prevent any radiative exitance from the surface because of its opposing radiance. This is an electromagnetic property or state. This has nothing to do with any thermodynamic process”)
“Thermodynamic processes cause non radiative air to be warmer than the cold of space in the first place.”
OK no problem.
“As I keep telling you, the radiative fluxes within an atmosphere are the consequence of non radiative thermodynamics and not the cause.”
Such is your fantasy! Please try to learn somthing of electromagnetic radiation (flux) that has nothing to do with thermo-anything, only the difference in radiance at each frequency. EMR is never heat, never thermodynamic.
Kristian said:
“Is the air above the surface warmer than the surface as soon as the Sun sets, Stephen? Meaning, over 50% of the globe at any specific time? ”
It also takes a while after sunrise for the surface to reach the temperature of the air above. It all balances out.
and:
“Are typical high latitude winter conditions present over 50% of the globe at any point in time? And is the air warmer than the surface wherever and whenever such winter conditions prevail?”
You can say the same in reverse about high summer conditions. It all balances out.
If at any time it doesn’t balance out you just get more wind as more warm air flows across colder surfaces or more cold air flows across warmer surfaces.
That is what weather is all about.
“electromagnetic radiation (flux) that has nothing to do with thermo-anything, only the difference in radiance at each frequency. EMR is never heat, never thermodynamic.”
The initial level of radiance is determined by thermodynamics.
Stephen Wilde says: September 17, 2014 at 11:30 am
(“If warm air were lying on top of a cool surface, then that warm air would indeed increase the HEATING of that surface, by transferring energy to it as HEAT. It wouldn’t reduce its COOLING.”)
“A surface beneath a clear sky radiates readily to space and cools the air above it in the process. It draws energy from the air by conduction. The energy drawn from the air results in the surface not cooling as fast as if there were no atmosphere.”
OK whatever you may mean by cooling, with no definition!
“If there is wind constantly replacing the energy drawn from the air by the radiating surface then the temperature fall for the surface will be even less.”
You are completly backward on energy drawn. EMR flux is not reliant on adjacent sensible heat
that heat transfer to that surface is what may sustain that EMR flux, at that temperature.
Stephen Wilde says: September 17, 2014 at 11:48 am
(Bryan, “Joules are merely a measure of the amount of work done with or against gravity in achieving the change in height.”)
“Joules are not a measure of internal energy.
http://dictionary.reference.com/browse/joule”
The Joule is “the” measure of any form of energy, internal, external, potential, electromagnetic, work, or any other form of energy that may come along. You spout only nonsense!!
Trick says: September 17, 2014 at 12:18 pm
(Will 4:53am: “There is no S-B case with a electromagnetically disbursive atmosphere in a gravitational field.”)
“Will is refuted by the top post picture analysis = test performed by radiosonde. Planck distribution and S-B therefore work as observed in an atmosphere.”
Nothing there has anything to do with any perfect S-B equation of the maximum. No Planck maximum distribution of anything. Only lying deceitful hand waving!
(6:17am: “What conservation of energy is required for an atmosphere that is free to dispatch any amount of entropy to space via adjustable EMR?”)
“In an atmosphere parcel, enthalpy is the conserved quantity by 1st law. Energy in – Energy out of parcel = change in internal energy of parcel. Entropy always increases in natural processes. Rather simple Will.”
What total bull shit, in this atmosphere entropy is obviously dispatched to elsewhere/elsewhen, via EMR flux. About this Earth there is never an increase in entropy! What a nice planet!
Kristian says: September 17, 2014 at 12:45 pm
Bryan says, September 17, 2014 at 11:54 am:
(“Stephen Are you taking the piss?”)
“I think he is.”
What wierd critters. Is “taking a piss” similar to “having sex”? In what possible way?
Pissing and screwing are much more informative!
Will Janoschka
‘Taking the piss’ is a UK term someone having a laugh at others expense.
Playing a joke to wind everybody up.
Stephan wants to talk about State Functions, Internal Energy,the Laws of Thermodynamics Adiabatic Atmosphere and so on yet does not know that the Joule is a unit of energy.
Well Steven were all in on the joke now.
No atmosphere, no potential energy, only kinetic energy at the surface.
Add a non radiative atmosphere which must show convection because surface heating is never even.
With convection comes creation of potential energy stored within the atmosphere.
That potential energy is created from surface kinetic energy which would otherwise have radiated to space. The surface temperature does not drop below S-B during the creation process because conduction is slower than radiation, not faster. A slower process cannot remove energy faster than it arrives.
The amount of potential energy which accumulates will be proportionate to the amount of work against gravity required to lift the mass of the atmosphere off the surface and hold it off the surface.
Since an adiabatic process is a closed system the potential energy within cannot escape to space.
Instead, it warms the surface of Earth by 33C because it is constantly being returned to the surface as KE in the descent phase.
Add radiative gases.
They allow radiation to space from within the atmosphere.
That means less potential energy in the descent phase of adiabatic overturning and less reconversion of PE to KE at the surface.
Radiation to space from within the atmosphere is exactly equal to the reduction in potential energy returning to the surface for a net zero thermal effect for the system as a whole.
The reduction in returning PE to KE at the surface exactly offsets the increased DWIR from GHGs for a net zero thermal effect at the surface.
“does not know that the Joule is a unit of energy.”
Duly corrected. Makes no difference.
Stephen Wilde says: September 17, 2014 at 1:41 pm
Bryan,
“I take the point about Joules but found this:
“In thermodynamics, the internal energy is one of the two cardinal state functions of the state variables of a thermodynamic system. It refers to energy contained within the system, while excluding the kinetic energy of motion of the system as a whole and the potential energy of the system as a whole due to external force fields. It keeps account of the gains and losses of energy of the system.” http://en.wikipedia.org/wiki/Internal_energy”
WOW A true attempt by the post modern idiots, with the kinetic theory of everything, to try to confuse all who are not arrogant academics. Does not work! State functions my ass! Thermodynamics with all its warts is complete in 1903. Like the latin language, no improvement is desired, we understand the warts, we wish that, that does not change by wishes of some fool!
The wish here is to confound various forms of heat energy that has no vector momentum. how about the energy in your car battery. Is that heat? It must be internal but is distinct from heat, and that 15 kg battery can have much Newtonian kinetic energy while driving at 50km/hr. Can we not at least try to make our words clearly understandable to others, or are all trying to scam all others?
Bryan says:
September 17, 2014 at 3:27 pm
Will Janoschka
‘Taking the piss’ is a UK term someone having a laugh at others expense.
Playing a joke to wind everybody up.
Stephan wants to talk about State Functions, Internal Energy,the Laws of Thermodynamics Adiabatic Atmosphere and so on yet does not know that the Joule is a unit of energy.
Well Steven were all in on the joke now.
———————————————————————
Thank you Bryan,
What ever is the UK term for “having sex”? I am not a sheep!
Will 2:48pm: “Nothing there has anything to do with any perfect S-B equation of the maximum. No Planck maximum distribution of anything.”
The top post picture was constructed from Planck distribution and S-B analysis. The result agrees with radiosonde test data. Your premise is refuted.
“About this Earth there is never an increase in entropy!”
There is no hope for Will writing against the 2nd law of thermodynamics.
******
Stephen 3:29pm: “Instead, it warms the surface of Earth by 33C because it is constantly being returned to the surface as KE in the descent phase.”
It?? To determine the DALR, consider the rate of decrease of temperature with height of a parcel of air that ascends (and descends) in such a way that its pressure is always that of the surrounding air. During this ascent (and descent) the parcel does not exchange any energy with its surroundings by virtue of a temperature difference between them (hence “adiabatic”), and any water vapor the parcel may contain is not transformed into liquid or ice (hence “dry”). Thus no adiabatic parcel can “warm the surface of the earth”.
Stephen continuously misses this science when he writes descending parcels warm the surface 33C. That Stephen misses this science is confirmed by the picture in the top post analysis=data.
Will Janoschka says, September 17, 2014 at 1:24 pm:
“Be careful of the word “heating”. This is a ClimAstrology weasel word. Increasing the sensible heat of that mass is what you mean, I think.”
No, of course that’s not what I mean. You cannot increase ‘heat’ in a mass. You increase the internal energy [U] of that mass. In thermodynamics, heat is some of that energy being transferred from that mass to another region by virtue of that mass being hotter than the other region. All the ‘heat’ that you ever ‘sense’ comes from energy being transferred from a place hotter than you.
‘Heating’ is not a ‘climastrology weasel word.’ It is a common word describing an increase in internal energy [U] of a system/region by transferring energy to that system/region, making it warmer in the process. ‘Cooling’ is likewise a term describing a decrease in internal energy of a system/region by transferring energy from that system/region, making it cooler in the process.
Normally when talking about ‘heating’ and ‘cooling’, one refers to a thermal process, that is, the energy is transferred to/from the system/region AS HEAT [Q].
A system can however warm and cool without a transfer of energy as heat also. For example in an adiabatic process. Then the transfer of energy comes about by work [W] being done by/on the system.
So the terms ‘heating’ and ‘cooling’ are not really precise in a strictly physical setting. They depend on the context being known.
Will Janoschka says, September 17, 2014 at 3:43 pm:
“WOW A true attempt by the post modern idiots, with the kinetic theory of everything, to try to confuse all who are not arrogant academics. Does not work! State functions my ass! Thermodynamics with all its warts is complete in 1903. Like the latin language, no improvement is desired, we understand the warts, we wish that, that does not change by wishes of some fool!
The wish here is to confound various forms of heat energy that has no vector momentum. how about the energy in your car battery. Is that heat? It must be internal but is distinct from heat, and that 15 kg battery can have much Newtonian kinetic energy while driving at 50km/hr. Can we not at least try to make our words clearly understandable to others, or are all trying to scam all others?”
Could you perhaps start with yourself, Will? Even after having been told a hundred times in a straightforward and consistent manner how ‘internal energy’ [U], ‘heat’ [Q] and ‘work’ [W] are strictly defined and used in thermodynamics, you refuse to do anything but mix them all into one mindless jumble, eyes tightly closed, fingers deep in ears, screaming your Scam! Scam! Scam! mantra. That’s what you do. And then you accuse everyone else of spreading confusion and trying to dupe all you noble creatures into letting go of your 1903 ‘knowledge’.
Again, what does the 1st Law of Thermodynamics defined for a closed thermodynamic system mean to you, Will?
ΔU = Q – W
How do you define U, Q and W? What is this equation actually saying in your world?
When the field of thermodynamics specifically describes ‘internal energy’ [U] as the quantity which ‘keeps account of the gains and losses of energy of the system’, what does this tell you about thermodynamic processes? The difference between state and process, between a static function and a dynamic function?
Kristian says: September 17, 2014 at 4:48 pm
Will Janoschka says, September 17, 2014 at 3:43 pm:
(“WOW A true attempt by the post modern idiots, with the kinetic theory of everything, to try to confuse all who are not arrogant academics. Does not work! State functions my ass! Thermodynamics with all its warts is complete in 1903. Like the latin language, no improvement is desired, we understand the warts, we wish that, that does not change by wishes of some fool!
The wish here is to confound various forms of heat energy that has no vector momentum. how about the energy in your car battery. Is that heat? It must be internal but is distinct from heat, and that 15 kg battery can have much Newtonian kinetic energy while driving at 50km/hr. Can we not at least try to make our words clearly understandable to others, or are all trying to scam all others?”)
“Could you perhaps start with yourself, Will? Even after having been told a hundred times in a straightforward and consistent manner how ‘internal energy’ [U], ‘heat’ [Q] and ‘work’ [W] are strictly defined and used in thermodynamics, you refuse to do anything but mix them all into one mindless jumble, eyes tightly closed, fingers deep in ears, screaming your Scam! Scam! Scam! mantra.”
Interesting Kristan, Please explain how your 2002 neavuo science is in any way better than the thermodynamic 1903 ‘knowledge’.”
“Again, what does the 1st Law of Thermodynamics defined for a closed thermodynamic system mean to you, Will? ΔU = Q – W How do you define U, Q and W?
What is this equation actually saying in your world? ”
Your equation is a fantasty created by kinetic everything idiots, to promote some conservation of energy with no appropriate Lorentz transform to include mass. We all know now how to convert mass into energy. We have no clue as to convert energy to mass and what may be the waste products.
Your delta U is not some internal energy it is an abstract conversion of usless heat energy (Q) divided by the the lowest temperature, (entropy) your U is never internal energy it is only the local accumulated entropy. Q-W, and can only be dispatched to lower radiance via EMR flux.
“When the field of thermodynamics specifically describes ‘internal energy’ [U] as the quantity which ‘keeps account of the gains and losses of energy of the system’, what does this tell you about thermodynamic processes?”
It means to me some artificial bookkeeping with no relevance to anything physical. It is a fantasy and a fraud!
Kristian says:
September 17, 2014 at 4:25 pm
A system can however warm and cool without a transfer of energy as heat also. For example in an adiabatic process. Then the transfer of energy comes about by work [W] being done by/on the system.
This applies to a closed system with, constant g and T.Such as the gas in a piston cylinder of a steam, diesel or petrol engine.it does not apply to the atmosphere
http://encyclopedia2.thefreedictionary.com/Barometric+Formula
The real distribution of air pressure and density in the earth’s atmosphere does not obey the barometric formula, since within the boundaries of the atmosphere the temperature and acceleration of free fall vary with altitude and geographic latitude. In addition, the atmospheric pressure increases with the concentration of water vapor in the atmosphere.
The atmosphere is not barometric it is hydrostatic.
The hydrostatic assumption assumes Newton’s 2nd law. It does not assume ideal gas law.
Barometric Formulas, require Ideal gas law conditions
1.Enclosed
2.Small volume
3.Temperature, pressure and density homogeneous
4 Temperature and pressure near STP.
4.Constant gravity
The atmosphere breaks these in spectacular fashion
1. Open at top and sides
2. 10^10 km^3
3.Temperature, pressure and density vary
4.Temperature and pressure go very far from STP
5.Gravity varies vertically
Air packets rising, expanding and doing work
LR is everywhere so air packets must be everywhere.All packets must expand so there is not enough space.
How can air packets all do work on each other?
Where does the energy go?
How is work done against falling pressure?
Roger Clague what point are you trying to make about the barometric formulas?
In might be better to use a full derivation such as the G&T link below to illustrate your objection rather than the summary link you supplied
Nobody is claiming the status of a law for these equations
G&T point out in the text
” At this point, it is important to remember that the
barometric formulas do not hold globally but have only a limited range of validity.”
However they do use the laws of thermodynamics and the kinetic theory of gases in their derivation
The equations are derived for an isothermal atmosphere and an adiabatic atmosphere in G&T link
This does not preclude further refinements as your link suggests but they are all collectively known as the barometric equations
Click to access 1003.1508.pdf
Even your link supplies what would be adequate accuracy for most purposes
” In this case the barometric formula is written in the form Δh = 18,400(1 + ɑt)log(p1/p2) (in meters), where t is the average temperature of the layer of air between the measuring points and α is the temperature coefficient of the volumetric expansion of air. The error for calculations with this formula does not exceed 0.1–0.5 percent of the altitude being measured.”
WIll Says: “Please explain how your 2002 neavuo science is in any way better than the thermodynamic 1903 ‘knowledge’.”
Sigh …
1) The equation you are complaining about — ΔU = Q – W — is one of the cornerstones of CLASSICAL pre-1903 thermodynamics! It is the statement of conservation of energy within the scope of classical thermodynamics. It has NOTHING to do with “neavuo” kinetic theory or statistical mechanics. I bet this equation is in the book you used to learn thermodynamics!
For example, Clausius in the mid-1800’s (ie before ‘neavuo science’) said “In a thermodynamic process involving a closed system, the increment in the internal energy is equal to the difference between the heat accumulated by the system and the work done by it.” This is exactly ΔU = Q – W
2) The mass-energy equivalence that you wanted to include is NOT part of classical pre-1903 thermodynamics. Einstein did not even start to develop this concept until 1905, well after you claim thermodynamics was completed.
**************************************
Basically, things you want to complain about as being “neavuo” are old, and things you include as old, are ‘neavuo”.
Tim Folkerts says: September 17, 2014 at 8:20 pm
(WIll Says: “Please explain how your 2002 neavuo science is in any way better than the thermodynamic 1903 ‘knowledge’.”)
“1) The equation you are complaining about — ΔU = Q – W — is one of the cornerstones of CLASSICAL pre-1903 thermodynamics! It is the statement of conservation of energy within the scope of classical thermodynamics. It has NOTHING to do with “neavuo” kinetic theory or statistical mechanics. I bet this equation is in the book you used to learn thermodynamics!”
Yes but U is entropy not some neuvo “internal energy”. It is waste energy no longer Q as it can do no work W.
“For example, Clausius in the mid-1800’s (ie before ‘neavuo science’) said “In a thermodynamic process involving a closed system, the increment in the internal energy is equal to the difference between the heat accumulated by the system and the work done by it.” This is exactly ΔU = Q – W”
Rudy never called that the neuvo “internal energy” He reluctantly agreed with “entropy” although that is a fake construct “waste heat” divided by some arbratry temperature. That fake construct was used rather than “useless Q” to prolong that illusion of “conservation of energy”
“2) The mass-energy equivalence that you wanted to include is NOT part of classical pre-1903 thermodynamics. Einstein did not even start to develop this concept until 1905, well after you claim thermodynamics was completed.”
Indeed. I want no e=mc^2 that is even more stupid than entropy. The idea of squaring an asymptote just screams “wrong” How to approach a surface with multiple velocities and never get there? You arrogant academics have much to explain to all, before nailing to the cross.
“Basically, things you want to complain about as being “neavuo” are old, and things you include as old, are ‘neavuo”.
The “neavuo” is but arrogant academics trying to cover up ancient mistakes with more bullshit, like “internal energy” without Newtonian, chemical, or electrical energy! Up on the cross!
@Stephen Wilde, September 15, 2014 at 3:49 pm
“That sounds rather as though it is mass density that controls the transparency of an atmosphere to infrared”
I think we are on the same team but somehow we are not communicating effectively. According to Robinson & Catling the IR optical depth in the stratosphere is directly proportional to pressure (Doppler broadening dominates). In the troposphere the optical depth is proportional to pressure squared owing to pressure-broadening and collision induced absorption (in layman’s terms a “Double Whammy”). The tropopause is the transition region. Here is a quote from R&C (2014):
“Our model uses a known power law between pressure (p) and the grey infrared optical depth of τIR ∝ pn (ref. 10). This scaling arises from combining the differential optical depth dτIR = −κρa dz (the differential altitude) with hydrostatic equilibrium dp/dz = −g ρ (where g is the gravitational acceleration and ρ is atmospheric density), so that dτIR ∝ κdp (see Supplementary Information). Below middle stratospheres, radiative transfer is dominated by pressure-broadening and collision-induced absorption, which have κ ∝ p, and, thus, n = 2 from integration (ref. 11) . We do not use n = 1, which would correspond to higher levels of the atmosphere where Doppler broadening dominates (ref. 12,13) and κ is independent of pressure (see also Supplementary Fig. 3).”
R&C are explaining temperature gradients in planetary atmospheres in terms of PRESSURE rather than the concentration of gases such as CO2. Eat your heart out James Hansen with your loony CO2 driven “Runaway Greenhouse Effect”.
If you look at the numbers R&C’s pressure based model fits observations while the Arrhenius (1896) hypothesis does not. The correlation between [CO2] and Antarctic temperatures over the past 800,000 years is a consequence of Henry’s Law rather than doublings or halvings of CO2 concentration.
gallopingcamel 4:24am: “R&C are explaining temperature gradients in planetary atmospheres in terms of PRESSURE rather than the concentration of gases such as CO2.”
No. R&C specifically explain temperature gradients in planetary atmospheres including pressure AND the concentration of gases such as CO2 in dτIR ∝ κdp where κ is the grey “opacity (e.g., various absorbing gases)”. See p. 8 eqn. S12. This is confirmed by the top post picture analysis=test.
Ditto what Trick just said. It is not “pressure rather than concentration”, it is “pressure in conjunction with concentration”.
The theory has always been about how the outgoing IR looks from the top, which depends on the thickness of the atmosphere and the gases that absorb/emit IR.
Bryan says:
September 17, 2014 at 7:23 pm
However they do use the laws of thermodynamics and the kinetic theory of gases in their derivation
The equations are derived for an isothermal atmosphere and an adiabatic atmosphere in G&T link
Click to access 1003.1508.pdf
G and T claim to have a model of the atmosphere which explains why LR = T/h = g/c
This model, like all models, contains assumptions ( postulates ). The postulates I say are not reasonable are
1.The air of the atmosphere obeys an equation of state of an ideal gas.
2.We postulate an isothermal atmosphere.
3.For the ideal gas we use the reversible work form p dv
Also but not stated
4.Air packets cannot gain energy ( adiabatic)
1.I have given reasons why gas laws cannot be applied to the atmosphere.
2.The paper postulates an isothermal atmos. yet the result of the model is that the atmos. has a temp. gradient.
3.
(a)Air packets do not expand if the atmos. is isothermal.
(b)Air packets cannot expand because the packets next to it are also trying to expand
(c) Air packets do not expand or do work on other air packets
4.Air packets get energy from collision of energised WV with N2 and O2
My objection this paper are
1. That all the thermodynamic postulates listed are not reasonable. The only thermodynamic postulate I accept is the 1st law of conservation of energy.
2. The postulates are contradictory. Air cannot be isothermal and abiabatic at the same time.
3.LR = T/h = g/c can be got by only assuming
(a) The hydrostatic equation
(b) KInetic Energy = gravitational energy
mgh = mcT
T/h = g/T
Also consider:
Using specific heat of air > LR = 10K/km
Using specific heat of WV > LR = 5.3K/km
measured av. world LR = 6K/km
Time for new thinking on LR. The adiabatic theory is 100 years old.
Roger says: “2. The postulates are contradictory. Air cannot be isothermal and abiabatic at the same time.”
They are considering two separate models — one that is isothermal and one that is adiabatic. They are not assuming both at once in a single atmosphere.
Roger 4:39pm: ”Time for new thinking on LR.”
No Roger – Tim F. 5:30pm is correct. Roger just needs to read the paper closely & correctly. G&T are simply parroting Poisson over 100 years ago only in section 2.4 for their postulated adiabatic atmosphere. This has stood the test of time.
”The postulates are contradictory. Air cannot be isothermal and abiabatic at the same time.”
Correct Roger and that is just what G&T point out. In section 2.3 they calculate density rho(z)=function(z) for an isothermal atmosphere and then go on in section 2.4 and find Poisson’s relations for the adiabatic atmosphere. As they write: ”These adiabatic equations of state are well-known from standard textbooks” at least ever since Poisson figured them out.
”The postulates I say are not reasonable are : 1. The air of the atmosphere obeys an equation of state of an ideal gas.”
This is accurate Roger as reference to any weather station data that includes measurements for temperature, density, pressure at the site – a quick glance will show for the air of the atmosphere p=density*R*T i.e. IGL is not observed in station data. Sometimes p goes up while temperature and density go down! in the data taken over time.
However once one idealizes to the parcel of air, then that parcel must theoretically follow p=density*R*T IGL coherence as those measurements are observed in close enough accord with IGL to be useful. This is in part also confirmed by the top post picture analysis=data.
Tim Folkerts says
“They are considering two separate models — one that is isothermal and one that is adiabatic.”
I agree
Roger Clague says:
“Time for new thinking on LR. The adiabatic theory is 100 years old.”
There is always room for progress but as it stands the various versions of the barometric formulas are the best we’ve got.
Even beating GPS for altitude
http://www.pasco.com/support/technical-support/technote/techIDlookup.cfm?TechNoteID=498
For certain conditions Van Der Waals Equation is more accurate than Ideal Gas Equation
http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/waal.html#c1
So as usual whats acceptable in the way of accuracy changes with demands and conditions
Roger Clague says:
“(b) KInetic Energy = gravitational energy”
Perhaps I mistake your point but you seem to imply by this that in the case of an air parcel it loses KInetic Energy which turns into Gravitational Potential Energy
Now G&T use the hydrostatic condition (and by the way this is more related to Newtons First Law than the Second).
Ist Law
Which is a body will remain at rest or move with constant velocity unless acted on by an unbalanced force.
In the case of an air parcel the weight acting down mg is balanced by buoyancy force acting up.
The buoyancy force arises from the pressure at top of parcel being less than on the bottom
So if the parcel has the slightest motion upward it will continue upwards without losing any internal energy being directly turned into potential energy.
Up Thread I did a worked example for Stephan showing this.
.
Where the upward parcel loses internal energy is expanding against its surroundings .
Compressing them thus doing PdV work
This energy is stored in the surroundings.
I chose STP and 1000m as these values are well know for DALR.
If Stephen (and perhaps yourself ) had been correct then internal energy lost by a mole of air in going from STP to 1000m would equal PE gained at that height.
It is nowhere near this value.
Instead the internal energy lost is exactly equal to the work done calculated from one of the barometric equations
[…] Hockey Schtick: CO2 does what exactly? […]
Roger says: “2. The postulates are contradictory. Air cannot be isothermal and abiabatic at the same time.”
Tim F says
They are considering two separate models — one that is isothermal and one that is adiabatic. They are not assuming both at once in a single atmosphere.
G and T say
Click to access 1003.1508.pdf
scetion 3.results
By combining hydrodynamics, thermodynamics, and imposing the above listed assumptions
for planetary atmospheres one can compute the temperature profiles of idealized atmospheres.
There is one derivation of 58 equations of LR = g/c.
assumptions:
1-5 get us to p = rhogh, the hydrostatic equation
6. IGL
7a. isothermal
7b. reversable work = pdv
These are part one continous argement using in turn all of these assumptions.
They are different theories but they are combined, wrongly in the derivation of LR = g/c by G and T.
Roger Clague
Its good to see you have found your way back to the correct thread.
Start the adiabatic section on page 8
Where do you find G&T using an isothermal assumption?
It surely is too much to expect that G&T would give an explicit warning to readers that this now implies that the constant temperature condition is now dropped.
Perhaps you might have liked such a warning.
G&T on the other hand have to assume a certain level of reading comprehension on behalf of the readers or their paper would be several pages longer.
Bryan says:
September 19, 2014 at 3:56 pm
Where do you find G&T using an isothermal assumption?
It’s your theory. O.K.The isothermal condition is dropped for 2.4.
It surely is too much to expect that G&T would give an explicit warning to readers that this now implies that the constant temperature condition is now dropped.
It is too much to expect me to know that conditions will be introduced in a model and then dropped. Why introduce a condition T constant and then drop it? Finally to reach a conclusion that T varies. This is opposite to the condition you have introduced, not used and then dropped?
.G&T on the other hand have to assume a certain level of reading comprehension on behalf of the readers or their paper would be several pages longer
I think what G&T assume is that if they make their derivation complex enough no-one will look at it closely.And spot the flaws.
Let’s ignore the strange disappearance of the isothermal condition. Let us look at:
2.4 The Adiabatic Atmosphere
Assumption 7b, for the ideal gas we use the reversible work form p dv
dv means the volume is changing
Your molar volume ( 22l = 30cm^3 ) is expanding ( or contracting ).
LR is everywhere, so your air packets should be do work everywhere. the model must explain LR everywhere on the Earth’s surface. So the 30cm^3 cubes are surrounded by others which are also must expand and do work to lose energy.
This is not possible. There is not enough space and no where for the energy to go.
It goes into gravitational potential energy.
Where do equations (22), (23), (24), three relations for the heat differential form dQ, come from?
Is it this?
http://en.wikipedia.org/wiki/Heat_equation
Which appears to apply to solids not gases.
Latent heat
The claim is that latent heat reduces LR from 10K/km to the normal range of 5-6.5K/km.
No latent heat over a desert, but LR = 6.5K/km over desserts.How?
Roger Clague
What do you mean by
“Your molar volume ( 22l = 30cm^3 )”
Do you not know that 22 litres = 22000 cubic centimetres?
If thats the case then you will not understand anything as complicated as an isothermal or adiabatic atmosphere.
Take a basic course in science is my best advice.
Roger say: “They are different theories but they are combined, wrongly in the derivation of LR = g/c by G and T.”
The way I read this, the derivation of LR = g/c is within Section 2.4 (Adiabtic Atmosphere) which uses Assumption 7b (reversable work) . No where in this section are they using the isothermal Assumption 7a — in fact they are now explicitly including dT and integrals with respect to dT. Where do you see them combining the isothermal assumption from the previous section with the adiabatic assumptions in this section?
Bryan wonders: “What do you mean by
“Your molar volume ( 22l = 30cm^3 )””
I suspect that this was a case of careless parentheses (and rounding off a bit much).
22000 cm^3 = (28 cm)^3
If you round off a bit from 28 to 30, then a cube about 30 cm on a side is about 22000 cm^3 = 22 l. So the original almost surely meant ‘about (30cm)^3’ where the parentheses were left out.
Bryan says: September 19, 2014 at 6:23 pm
“Roger Clague What do you mean by”
(“Your molar volume ( 22l = 30cm^3 )”)
“Do you not know that 22 litres = 22000 cubic centimetres?”
Here I must agree with Tim Folkert,
22000 cm^3 = (28 cm)^3, That same “mole” STP, hardly a noticeable part of the atmosphere!
“If thats the case then you will not understand anything as complicated as an isothermal or adiabatic atmosphere. Take a basic course in science is my best advice.”
I do not agree with Roger Clague’s analysis or your implied claim, of isothermal “or” adiabatic!
Please explain why the atmosphere cannot be both? A “gravitationly induced temperature/ pressure gradiant”, a thermostatic property, maintained without regard to any thermodynamic process.
This results in an “isotherm” with an externally maintained temperature gradiant for any tropospheric thermodynamic process. If such cannot accepted, Please explain the re-thermalization of the 10 km troposphere with a 6 minute time constant? This is way to rapid for atmospheric mass transport (strict thermodynamic convection). Why must colloquial theory trump understanding?
Will Janoschka says
“Here I must agree with Tim Folkert,
22000 cm^3 = (28 cm)^3, That same “mole” STP, hardly a noticeable part of the atmosphere!”
Where the hell did the 28 litres come from?
It is school pupils knowledge that a mole of any gas occupies a volume of 22.4 litres at STP!
I can only assume that Tim Folkerts was bending over backwards to try to rescue some sense from Rogers post but this amount of ’rounding off’ is not acceptable.
Will Janoschka says
“Please explain why the atmosphere cannot be both? A “gravitationly induced temperature/ pressure gradiant”, a thermostatic property, maintained without regard to any thermodynamic process.”
Perhaps you would like to read what Maxwell said on the topic
After a lecture on why the Ideal Gas Equations imply that in a flat Earth surface uniformly illuminasted by the Sun an isothermal atmosphere is the result
However
”This result is by no means applicable to the case of our atmosphere. Setting aside the enormous direct effect of the sun’s radiation in disturbing thermal equilibrium, the effect of winds in carrying large masses of air from one height to another tends to produce a distribution of temperature of a quite different kind, the temperature at any height being such that a mass of air, brought from one height to another without gaining or losing heat, would always find itself at the temperature of the surrounding air. In this condition of what Sir William Thomson has called the convective equilibrium of heat, it is not the temperature which is constant, but the quantity ϕ [entropy], which determines the adiabatic curves.
In the convective equilibrium of temperature, the absolute temperature is proportional to the pressure raised to the power (γ-1)/γ, or 0,29.
The extreme slowness of the conduction of heat in air, compared with the rapidity with which large masses of air are carried from one height to another by the winds, causes the temperature of the different strata of the atmosphere to depend far more on this condition of convective equilibrium than on true thermal equilibrium.”
UPDATE: Maxwell’s statement “In the convective equilibrium of temperature, the absolute temperature is proportional to the pressure raised to the power (γ-1)/γ, or 0,29.” is referring to γ defined as the “heat capacity ratio” = Cp/Cv [ratio of specific heat capacity at constant pressure to specific heat capacity at constant volume]
Bryan says: September 20, 2014 at 8:50 am
Will Janoschka says
( “Here I must agree with Tim Folkert,
22000 cm^3 = (28 cm)^3, That same “mole” STP, hardly a noticeable part of the atmosphere!”)
“Where the hell did the 28 litres come from?”
What 28 litres? Bryan!
22.4 litres fits into a cube, how many centimeters in each side? A container for a mole at STP!
“It is school pupils knowledge that a mole of any gas occupies a volume of 22.4 litres at STP!”
Please demonstrate that the same school pupils have any concept of the “mole” “22.4 litres”, or STP? Do you have any such concept? Please clarify your knowledge?
Bryan says: September 20, 2014 at 9:07 am
(Will Janoschka says
“Please explain why the atmosphere cannot be both? A “gravitationly induced temperature/ pressure gradiant”, a thermostatic property, maintained without regard to any thermodynamic process.”)
“Perhaps you would like to read what Maxwell said on the topic”
”This result is by no means applicable to the case of our atmosphere. Setting aside the enormous direct effect of the sun’s radiation in disturbing thermal equilibrium, the effect of winds in carrying large masses of air from one height to another tends to produce a distribution of temperature of a quite different kind, the temperature at any height being such that a mass of air, brought from one height to another without gaining or losing heat, would always find itself at the temperature of the surrounding air. In this condition of what Sir William Thomson has called the convective equilibrium of heat, it is not the temperature which is constant, but the quantity ϕ [entropy], which determines the adiabatic curve”
Indeed. exactly! Entropy/enthalpy is involved, as the adiabatic depends on the properties of each gas under gravitational attraction, not with any heat transfer process. This is where Maxwell agreed with Lowschmidt that Earth’s atmosphere has a thermostatic gravitational gradiant of temperature and pressure. However for thermo dynamic process the troposphere is isothermic, with high thermal conductivity, as nothing else can explain the rapid maintainance of ALR equilibrium, independent of any thermodynamic process. All scienterrfic earthlings still cannot explain that. They do not try to explain! They all prefer BS.
Jimmy, Billy, Josef, Luddy, and Max did not make stupid mistakes.
Please explain why the atmosphere cannot be both adiabatic and diabatic at the same time? A “gravitationly induced temperature/ pressure gradiant”, a thermostatic property, maintained without regard to any thermodynamic process. This results in an “isotherm” with an externally maintained temperature gradiant for any tropospheric thermodynamic process. If such cannot accepted, Please explain the re-thermalization of the 10 km troposphere with a 6 minute time constant? This is way to rapid for atmospheric mass transport (strict thermodynamic convection). Why must colloquial theory trump understanding?
Will Janoschka says
“Please demonstrate that the same school pupils have any concept of the “mole” “22.4 litres”, or STP? Do you have any such concept? Please clarify your knowledge?”
If you have any further difficulty please let me help
http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/idegas.html
Rogers original
“Your molar volume ( 22l = 30cm^3 )”
Tims reinterpretation
22000 cm^3 = (28 cm)^3,
Why 28? Your guess is as good as mine on second thoughts perhaps its to do with the Atomic Weight of Nitrogen?
Bryan says: September 20, 2014 at 10:39 am
(Will Janoschka says“Please demonstrate that the same school pupils have any concept of the “mole” “22.4 litres”, or STP? Do you have any such concept? Please clarify your knowledge?”)
“If you have any further difficulty please let me help
http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/idegas.html”
[snip]
—————————————————————————————
Rogers original
“Your molar volume ( 22l = 30cm^3 )”
Tims reinterpretation 22000 cm^3 = (28 cm)^3,
—————————————————————————————-
“Why 28? Your guess is as good as mine on second thoughts perhaps its to do with the Atomic Weight of Nitrogen?”
[snip] has no concept of how to translate a specific volume into the same volume of a cube with a constant length along each and every edge!
Perhaps Tim Folkerts is very schmardt in comparison [snip]
[Moderation note] Stop insulting my contributors or go elsewhere. – TB
Bryan says, September 20, 2014 at 10:39 am:
“Rogers original
“Your molar volume ( 22l = 30cm^3 )”
Tims reinterpretation
22000 cm^3 = (28 cm)^3,
Why 28? Your guess is as good as mine on second thoughts perhaps its to do with the Atomic Weight of Nitrogen?”
I think Folkerts is on to something here, Bryan. Although only Clague himself could ultimately verify this.
A cube with sides 28cm x 28cm x 28cm would hold 22 litres. Folkerts is merely suggesting that Clague forgot his parentheses to make this point.
The reason I became interested in this topic was at originally I would have been inclined to agree with Stephen Wilde.
A rising air parcel gains GPE, surely this must come from a loss of its internal energy?
However on further investigation this turned out to be wrong.
Now to test the theory it is not too difficult to put in some numbers.
I picked a mole of air at STP out of convenience because most people know and accept the numbers given.
The other known temperature is at a height of 1000m on a dry atmosphere using the DALR
This would be 273K – 9.8K = 263.2K
I could have picked any mass of air and any height in the troposphere but that would have made it more difficult for others to check my numbers
Loss of internal energy for a mole of air at STP rising to a height of 1000m is 203J
Work done against surroundings is 203J (use of barometric equations)
So not one Joule of lost internal energy was turned into gravitational potential energy!
And yet the mole of air is now 1000m from the ground with this mass of air having apparently acquired 284J
The mole of gas at ground level would occupy 22.4 litres but at 1000m would have expanded considerably.
The air parcel RISES but an almost exactly similar parcel FALLS to replace the void left.
The falling parcel is to all intents exactly the same as the rising parcel
Rising one gaining PE and the falling one losing PE in exactly equal steps
The parcel at 1000m would lose exactly 243J of PE on returning to ground level.
The parcel would be compressed on the way down by its surroundings and thus would gain exactly 203J and its temperature would have risen to 273K and would occupy a volume of 22.4l.
The first law of thermodynamics has been satisfied in all respects.
Some ask why is there an adiabatic atmosphere?
A pocket of air is unlikely to have absolutely no vertical motion.
The hydrostatic condition also applies to motion at constant speed up and down
If less dense it will move up, if more dense it will move down.
Since PV/T is a constant it means that a warmer upward packet will expand slowly and drop temperature slowly to maintain hydrostatic equilibrium.
This is exactly the condition for adiabatic expansion.
(See the Carnot Cycle for further details)
Now back to the main point of this thread
What changes does a radiative gas fraction make to this situation?
For similar volumes of gases in the lower troposphere the effects are largely self cancelling
As the parcel moves higher to thinner air there will be a gradual greater loss through upward emission than absorption and an increasing heat transfer to space.
So our parcel would lose internal energy by this mechanism and start to descend as it is slightly denser than its surroundings.
All this is in line with the main article
Hi Bryan. What difference does the phase change of water make to your argument? In most places in the world, upward moving air is moister than downward moving air. Condensation removes significant volume from the air packet the water vapour resided in. Condensation also yields a lot of energy. Where does it go?
Tallbloke says
What difference does the phase change of water make to your argument?
This aspect I would only make a guess at, or simply quote some article.
So off the top of my head
Maximum water vapour fraction quoted is around 5%
But is it 5% of mass?
Lets go with that 5% mass of one mole of air
Latent heat released = mL = 0.05×0.022×2.3×10^6J = 2530J
This is a considerable amount of energy
If all this energy was used in raising the temperature of the air packet
2530 = SMdT where the specific heat capacity of air is around 1000 the mass is 22g
So dT would be about 115K which is unrealistic but perhaps more realistic if released more gradually dropping temperature adiabatically
Condensation will in itself reduce volume slightly keeping temperatures higher
The falling rain now reducing the mass making the parcel less dense
The consequences expected
Lapse rate now much lower .
The energy of the air packet continues to do work against its surroundings and that is where the bulk of the energy is transferred.
On the radiative aspect of condensation
Rain having a surface will radiate more effectively than a gass as it has a continuous IR spectrum.
Since this is happening near the top of the troposphere more cooling than heating will result.
Disclaimer
I fuly expect there are a number of errors in analysis above as I have been ‘winging’ it
Bryan thanks for ‘winging it’ for us. Sure enough the moist lapse rate is reduced from the DALR.
Your initial analysis allows for the fact that an air parcel near the surface will be subjected to higher pressure (and will therefore be at a higher temperature all else being equal) than air at altitude. But there is another factor here which is not captured by consideration of the situation as a classic ‘closed’ system first law equilibrium distribution.
Solar energy is entering and lw is leaving, and the higher density of the near surface air is resuting in a greater rate of interception of this radiated energy from the Sun and leaving the surface.
This must surely be a large part of the reason the air near the surface is warmer than the atmosphere at altitude? Does this not lead to a ‘surplus’ of energy near the surface which drives convection as part of the heat loss mechanism? And doesn’t that lead us to conclude that any notion of an ‘equilibrium atmosphere’ is false?
tallbloke says
“Does this not lead to a ‘surplus’ of energy near the surface which drives convection as part of the heat loss mechanism? And doesn’t that lead us to conclude that any notion of an ‘equilibrium atmosphere’ is false?”
Yes in particular the evaporation on water surfaces.
The dominant surface/solar effect is underrepresented in equilibrium calculations and implies more convective heating.
The effect of clouds simply by attaining a temperature and thus reducing convective cooling is underrepresented quite apart from radiative considerations.
However classical thermodynamics through the barometric equations gives a structure to the troposphere that can give useful information and experiments give broad support.
Given that they don’t involve any extrinsic radiative calculations (as the G&T paper shows) there is not much room left for a ‘greenhouse effect’.
Bryan says: September 20, 2014 at 3:33 pm
“Maximum water vapour fraction quoted is around 5%
But is it 5% of mass?”
See http://en.wikipedia.org/wiki/Relative_humidity#mediaviewer/File:Relative_Humidity.png
At 0C and 100% relative humidity the amount of water in a kg air is ~4g.
Fascinating to watch the process of good old meteorology being re-invented 😉
One question: where does the idea come from that the atmosphere could be adiabatic??
No energy entering or leaving the atmosphere?
Ben Wouters says:
“At 0C and 100% relative humidity the amount of water in a kg air is ~4g.”
Thanks that would reduce my figures to a great extent.
Ben asks: “One question: where does the idea come from that the atmosphere could be adiabatic??”
The “adiabatic lapse rate” assumes that a given “parcel” of air within the atmosphere can be treated as adiabatic as it moves within the atmosphere. For example, as a given 1 m^3 of air convects upward, neither conduction nor radiation can exchange much heat with surrounding parcels of air. But the air can expand as it rises. So to a decent approximation, the expansion is done with no heat exchanged.
Of course, at the bottom of the atmosphere there is heat input in the form of conduction from the ground, and at the top there is heat out in the form of radiation to space. So then the adiabatic assumptions break down.
Bryan, and at 30 degC and about 65% RH air is holding 20 g wv/kg air which is the c.a. 2% I see so often meantions as the global mean of water vapor held by our global atmosphere. Same for 35 degC at about 50% RH. Of course, most is held in the tropics.
Tim Folkerts says: September 20, 2014 at 8:24 pm
Thanks Tim, I think I have a solid understanding of the workings of the DALR and SALR regarding ascending or descending air parcels or masses in our atmosphere that is in hydrostatic balance with gravity.
What baffles me is when the temperature profile of the static (not vertically moving) atmosphere is attributed to these adiabatic processes. This is total nonsense. The atmosphere isn’t adiabatic as you noted.
A rising parcel does not exchange energy with its surroundings. It stops rising when its density (is ~temperature) is equal to the air it rises into. So where does this process influence the temperature profile of the static atmosphere?
The main effect of convection is to remove heat and WV from the surface, and bring it down again someplace else. If a lot of WV is brought high in the air it can radiate more easily to space, AFTER the convective phase, which lasts only minutes and can thus be considered adiabatic.
22.4l = 24 000cm^3
= 28cm^3
= 30cm^3 ( 1 sig.fig.)
Sorry to not make it very clear. However I was glad to see WillJ got it.
I was taught to approx. to 1 sig.fig. in rough work. To get a feel for whether your working and answers make sense.
Further thought on G and T 2010
Forget isothermal/adiabatic atmosphere and expansion/work done against pressure,
The main problem for me is the Ideal Gas Law (IGL)
The GPE/K.E. equation
mgh + mcT only has the variables we measure T and h. g for Earth and c(p) for the atmosphere are known. Mass cancels.The equation cannot be any simpler.
Including IGL leads to an 3 extra variables, R gas constant , M molecular mass and cp/cv ratio of specific heats.
They are not needed. They are not physically justified. They lead to fudging to get the required result.If you are interested I can show you were they pushed the maths to get the answer they were looking for.
T/h = g/c is damning evidence against CO2. Greens have banished it. But to get it back we need the correct derivation.
Click to access more.pdf
This guy puts G and T to shame. LR = 10K/km is found from 4 equations and without mentioning specific heat.
With four parameters I can fit an elephant, and with five I can make him wiggle his trunk.
Attributed to von Neumann by Enrico Fermi, as quoted by Freeman Dyson in “A meeting with Enrico Fermi” in Nature 427 (22 January 2004) p. 297
G and T have 5 variables.
Also g/c(p)N2 gets the wrong answer.
LR is not 10K/km over a dry area and is mostly 5-
6k/km.
g/c(p)H2O = 5K/km.
Should be:
24litre = 24 000cm^3
= (28cm)^3
= (30cm)^3
That is a cube of 30cm each direction. So you have some visual idea of the molar volume.
Bryan says:
September 20, 2014 at 1:37 pm
Loss of internal energy for a mole of air at STP rising to a height of 1000m is 203J
Work done against surroundings is 203J (use of barometric equations)
So not one Joule of lost internal energy was turned into gravitational potential energy!
It is your assumption that the loss of internal energy goes to work not GPE.
And yet the mole of air is now 1000m from the ground with this mass of air having apparently acquired 284J
Where did 284J come from? It gains the 203J GPE from the 203J K.E. lost
Evidence of atmos. not obeying gas Law.
Click to access angeo-19-1001-2001.pdf
fig.5
1800hr-2000hr the air is cooling at all levels. In the tropics so the sun is down. Yet the height of the tropopause is rising.This is the opposite of Ideal Gas Law prediction
Bryan
And yet the mole of air is now 1000m from the ground with this mass of air having apparently acquired 284J
I now realise the 284J is assuming the temp. drop is 10K. Better to calculate than assume.
1 mole air = 30g.
specific heat air =1J/gdegreeK
heat energy = mass x specific heat x dT
200J = 30g x 1J/gdegreeK x dT
dT = 200/30
dT = 7K
Roger Clague says:
“This guy puts G and T to shame. LR = 10K/km is found from 4 equations and without mentioning specific heat.”
Specific Heat is mentioned several times for instance on page 5.
γ ≡ Cp/CV
What do you think Cp and Cv are?
The Ohio derivation is fine as are all other competent presentations.
G&T were not claiming any special status for their derivation only to claim that they set out the boundary conditions more explicitly than most others.
But where you are really confused is to think that Gerlich and Tscheuschner are pushing a green agenda.
The reason that they published the paper was to show that the structure of troposphere can be described without separate radiative calculations .
Most readers here would know that G&T are considered to be hard line anti CO2 Greenhouse Effect critics .
Read their paper again or better still read their main paper
Click to access 0707.1161.pdf
.
Roger Clague says: September 21, 2014 at 3:56 pm
“Should be:
24litre = 24 000cm^3
= (28cm)^3
= (30cm)^3
That is a cube of 30cm each direction. So you have some visual idea of the molar volume.”
22.4litre = 22,400cm^3
= (28.18919493cm)^3
= (28cm)^3 less than 22 litre, still close.
approx (30cm)^3 = 27 litre, still close, for a non ideal gas like this atmosphere.
That is a cube of 30cm each direction. Very very close to “nothing/total atmospheric volume”.
Do not consider the velocity of one gas molecule when considering thermodynamics, and especially “never” when considering real gas thermostatics in a gravitational field.
Carefull of integers raised to high powers! The silencing of ClimAstrology 🙂
Bryan says:
September 21, 2014 at 5:28 pm
Specific Heat is mentioned several times for instance on page 5.
? = Cp/CV
What do you think Cp and Cv are?
Cp and cv are different properties, variables. The ratio of 2 variables is another different variable
gamma = cp/cv is not a specific heat.
Neither the words ” specific heat” nor the symbol for specific heat is mentioned.
But where you are really confused is to think that Gerlich and Tscheuschner are pushing a green agenda.
I said:
“T/h = g/c is damning evidence against CO2. Greens have banished it. But to get it back we need the correct derivation.”
I liked G and T’ 2009 falsification paper. I know they don’t think CO2 causes warming. But that is no excuse for incorrect physics in 2010.
The reason that they published the paper was to show that the structure of troposphere can be described without separate radiative calculations .
I think the structure of the troposphere is caused by radiation. Radiation exciting W.V.,H2O(g), not CO2.
I agree with G and T that CO2 does not cause warming but for a different reason.
Read their paper again or better still read their main paper
http://arxiv.org/pdf/0707.1161.pdf
This is a critique of the CO2 causes warming theory. It not about the lapse rate. Which we are discussing.
Roger Clague says
“Cp and cv are different properties, variables. The ratio of 2 variables is another different variable
gamma = cp/cv is not a specific heat.
Neither the words ” specific heat” nor the symbol for specific heat is mentioned.”
Read the table linked below
http://www.engineeringtoolbox.com/specific-heat-capacity-gases-d_159.html
Roger unfortunately I don’t have time to continue with dealing with elementary mistakes.
My best advice to you if as you seem interested in the topic is to revise over basic physics and then move onto thermodynamics then climate studies.
Roger Clague says: September 21, 2014 at 10:18 pm
Bryan says: September 21, 2014 at 5:28 pm
(“Specific Heat is mentioned several times for instance on page 5. ? = Cp/CV
What do you think Cp and Cv are?”)
“Cp and cv are different properties, variables. The ratio of 2 variables is another different variable
gamma = cp/cv is not a specific heat. Neither the words ” specific heat” nor the symbol for specific heat is mentioned.”
(“But where you are really confused is to think that Gerlich and Tscheuschner are pushing a green agenda.”)
“I said: “T/h = g/c is damning evidence against CO2. Greens have banished it. But to get it back we need the correct derivation.”
(“I liked G and T’ 2009 falsification paper. I know they don’t think CO2 causes warming. But that is no excuse for incorrect physics in 2010. The reason that they published the paper was to show that the structure of troposphere can be described without separate radiative calculations.”)
“I think the structure of the troposphere is caused by radiation. Radiation exciting W.V.,H2O(g), not CO2. I agree with G and T that CO2 does not cause warming but for a different reason.
Read their paper again or better still read their main paper
Click to access 0707.1161.pdf
This is a critique of the CO2 causes warming theory. It not about the lapse rate. Which we are discussing.”
Roger Clague, Only you are discussing lapse rate. The rest of us are struggling with “CO2 does what exactly”. The silencing of ClimAstrology 🙂
@Roger Clague,
“http://physics.ohio-state.edu/~jay/846/more.pdf
This guy puts G and T to shame. LR = 10K/km is found from 4 equations and without mentioning specific heat.”
Thanks for that link. A really elegant derivation of the DALR using only the diatomic nature of the main gases (nitogen & oxygen), gamma (γ), M & R.
However it does not ignore the concept of specific heat because:
Cp = M * (γ – 1) / γ / R
gallopingcamel says:
September 22, 2014 at 3:16 am
A really elegant derivation of the DALR using only the diatomic nature of the main gases (nitogen & oxygen), gamma (γ), M & R.
I was being sarcastic. I think they are both wrong, only the Ohio paper did it quicker.
Both have 5 variables, T,h, gamma, M and R, They can get any result they want.
However it does not ignore the concept of specific heat because:
Cp = M * (γ – 1) / γ /
I didn’t say they ignored it, I know they used it.
I said they did not mention it in the text. The words or the symbols for specific heat do occur.
Temp gradient = T/h = gravity/ specific heat
is toxic if you think CO2 cause warming.
Will Janoschka says:
September 21, 2014 at 11:58 pm
Roger Clague, Only you are discussing lapse rate. The rest of us are struggling with “CO2 does what exactly”.
Yes the post is about what CO2 does.
In particular, does CO2 cause warming?
So we need to discuss what causes warming of the atmos.
The warming of the atmos. is the vertical temp. gradient ( LR )
We need to know the cause of the LR
The 2 main theories are:
1. By convection of air by adiabatic cooling
2. By radiation exciting W.V. which heats the N2 and O2
The silencing of ClimAstrology
I want to engage with, not silence, anyone interested in applying science to understand the atmosphere.
We are all finding our way with a lot of new data about the atmosphere.
None of us has all the answers. Labels are misleading and not helpful.
Bryan says:
September 21, 2014 at 5:28 pm
But where you are really confused is to think that Gerlich and Tscheuschner are pushing a green agenda. and
Most readers here would know that G&T are considered to be hard line anti CO2 Greenhouse Effect critics .
I think it is wrong to read a science paper, such as G and T (2010) and also consider the political agenda of the authors, whatever it is. That was one of the main criticisms of Climategate.
The reason that they published the paper was to show that the structure of troposphere can be described without separate radiative calculations
So we must judge the paper on it sucess in do that.Not because they are on our side. I think it fails.
There are no sides in science.
gallopingcamel says: September 22, 2014 at 3:16 am
(@Roger Clague, “http://physics.ohio-state.edu/~jay/846/more.pdf
This guy puts G and T to shame. LR = 10K/km is found from 4 equations and without mentioning specific heat.”)
“Thanks for that link. A really elegant derivation of the DALR using only the diatomic nature of the main gases (nitogen & oxygen), gamma (γ), M & R.”
I agree “elegant”!!!
“However it does not ignore the concept of specific heat because: Cp = M * (γ – 1) / γ / R”
Also “elegant”!
When can we agree that this “is” consists of “all POVs” that point to the same puppy? We now have 57 individual POVs, each pointing to a different puppy! Poor mama dog! She knows not which to lick on the most! We are struggling with “CO2 does what exactly”. The silencing of ClimAstrology 🙂
Roger Clague says: September 22, 2014 at 9:56 am
Will Janoschka says: September 21, 2014 at 11:58 pm
(“Roger Clague, Only you are discussing lapse rate. The rest of us are struggling with “CO2 does what exactly”.)
“Yes the post is about what CO2 does.
In particular, does CO2 cause warming”
Indeed OK!
“So we need to discuss what causes warming of the atmos.
The warming of the atmos. is the vertical temp. gradient ( LR )
We need to know the cause of the LR”
Not at all! We can well measure lapse rate. Does atmospheric CO2 concentration change that whatsoever, as claimed by ClimAstrology?
“The 2 main theories are:
1. By convection of air by adiabatic cooling
2. By radiation exciting W.V. which heats the N2 and O2”
What total nonsense of claimed theories. Neither has anything to do with this atmosphere.
Way, way, way before any theories, is the measurement. How do your theories affect the measurement!
(“The silencing of ClimAstrology”)
“I want to engage with, not silence, anyone interested in applying science to understand the atmosphere. We are all finding our way with a lot of new data about the atmosphere.
None of us has all the answers.”
I agree! I wish to silence only the political BS of “we arrogant academics” already know everything, You must trust us, as we know. (religion). The silencing of ClimAstrology.
Bryan: However classical thermodynamics through the barometric equations gives a structure to the troposphere that can give useful information and experiments give broad support. Given that they don’t involve any extrinsic radiative calculations (as the G&T paper shows) there is not much room left for a ‘greenhouse effect’.
Thanks, and I agree. The level of radiative activity in the troposphere is more the outcome, than the cause of the temperature profile there, where evaporation and convection dominate the energy transport.
The radiative theorists have mistaken the radiative activity levels they are able to measure and fit their theory to, for the cause of the measured temperature profile.
This is where I disagree with Roger C and Tim F. So far, they’ve offered no coherent argument why we should accept the radiative hypothesis. It seems to rest on the mistaken belief that the two stream approximation refers to real and effective separate flows of energy in order to assert the greater magnitude of radiative activity over evaporative-convective activity.
If there really was a separate downwelling stream of 330W/m^2, we would be able to harness the energy, but we cannot.
tallbloke says: September 22, 2014 at 11:32 am
“If there really was a separate downwelling stream of 330W/m^2, we would be able to harness the energy, but we cannot.”
Roger,
That downwelling of whatever. Is what allows my doggy-dog to retreve another cold beer!
tallbloke 11:32am: “…no coherent argument why we should accept the radiative hypothesis.”
The top post picture is constructed entirely from radiative hypothesis analysis and found to match radiosonde data to within instrument error.
To understand physically why that is achieved by radiative transfer alone, one can learn the foundational basics then (hierarchically) move on to the two-stream theory from which one can acquire much of the physical intuition needed to understand multiple scattering. Truesdell’s history shows the best scientists have superb intuition (Faraday, Maxwell are examples).
If stop after having mastered the two stream model, one will have acquired much of the physical intuition behind that top post picture but not the final word (there is no final word). Intuition should show one that the 330 cannot be harnessed because of the nature of the radiation bath simply because this would have been done – then read why, that detail application of 2LOT prevents extracting useful work from the natural photon bath (Maxwell’s demon story).
Trick:
“the two-stream theory”
“the natural photon bath”
This is Trick trying to have his cake and eat it too.
You can’t have it both ways Trick. Either there are two separate streams of upwelling and downwelling longwave radiation and work can be extracted, or there’s a ‘photon bath’ and it can’t.
“The top post picture is constructed entirely from radiative hypothesis analysis and found to match radiosonde data to within instrument error.
To understand physically why that is achieved by radiative transfer alone,”
It isn’t acheived physically, only inside theoretical models. The facts show that the energies transported by the evaporation of water and the convection of the tropospheric air are bigger factors than radiation, which has a small net upward movement incapable of warming the surface except in rare localised circumstances.
The reason why the theoretical model mimics real measurements reasonably well is because it makes no difference to the magnitudes whether the radiation is the cause or the effect of the local temperature. The evidence is that it is more effect than cause. I’m able to assert that because I understand the relationships between density, heat capacity and latent heat Bryan and I discussed earlier.
The radiative greenhouse effect is tiny compared to the effect of gravity acting on atmospheric mass to generate higher pressure (and therefore density) near the surface. That more densely packed medium intercepts more solar energy and outgoing longwave from the ocean which absorbs the bulk of the solar energy. That’s why the surface air is warmer than air at altitude. Not because the near surface atmosphere is heated by ‘downwelling longwave radiation ‘trapped’ higher in the atmosphere, but because the radiation almost immediately thermalised in the dense near surface air is slow to convect and pass evaporated water upwards due to its density.
tallbloke says: September 22, 2014 at 2:56 pm
“The radiative greenhouse effect is tiny compared to the effect of gravity acting on atmospheric mass to generate higher pressure (and therefore density) near the surface. That more densely packed medium intercepts more solar energy and outgoing longwave from the ocean which absorbs the bulk of the solar energy. That’s why the surface air is warmer than air at altitude. Not because the near surface atmosphere is heated by ‘downwelling longwave radiation ‘trapped’ higher in the atmosphere, but because the radiation almost immediately thermalised in the dense near surface air is slow to convect and pass evaporated water upwards due to its density.”
Roger,
This is OK but still incorect.
“That more densely packed medium intercepts more solar energy and outgoing longwave from the ocean which absorbs the bulk of the solar energy.”
Not at all. The opposing radiance because of WV toward the surface stops any outgoing radiative flux. All waste heat in the atmosphere is via convection, and latent WV energy. That atmospheric waste heat (entropy) Is what is dispached to space, via EMR. never from Earth’s surface. The Earth’s surface is a piss poor way of radiating anything (measured)!
Tallbloke says: “If there really was a separate downwelling stream of 330W/m^2, we would be able to harness the energy, but we cannot.”
You don’t understand the 2nd Law if you make such a claim. Here are few brief arguments.
1) Consider a similar situation — air molecules bouncing around in a room. At any given moment, about 1/2 of the molecules are heading upward and about 1/2 are heading downward. There really, truly are “downwelling molecules” and other “upwelling molecules” at any given moment. Do you think we should be able to harness the energy of only the downwelling molecules?
2) If I put a colder object on the surface (say a liquid nitrogen cooled plate), then you COULD harness the 330 W/m^s of downwelling IR. This suggests the photons are there all along, but that the greater upwelling flow of photons prevents the effective use of these photons.
3) You also cannot harness the ~ 390 W/m^2 of downwelling IR — at least not all of it. Only the NET flow can be harnessed. And even more, the net flow is limited by the temperature differences.
hmmm … that might be a good challenge for people to contemplate. A 1 square meter 300 K object radiating to 282 K surroundings loses power at a rate of 100 W/m^2. So does a 250 K object radiating to 215 K surroundings. Or a 220 K object radiating to 155 K surroundings. But in the first case, we can only extract work at a rate of up to 6 W. For the second we could extract up to 14 W. For the last we could extract up 29 W. The amount of work that can be extracted depends not on the NET flow, but on the sizes of the upward and downward flows!
(In the end it doesn’t really matter. Proper application of either the opposing photon streams or net flux will lead to the correct answers.)
TB: “If there really was a separate downwelling stream of 330W/m^2, we would be able to harness the energy, but we cannot.”
And be very glad we can’t! Quite dangerous. In all essence that is not energy but a form of negative energy, that which counteracts and slows what energy ther is and can move from the surface to space, and if energy companies ever got a whiff of a method to somehow extract that negative energy I’m sure they soon would find a way to meter some of it into the transmission and transport lines to your home… charging us an extra charge in addition for what energy we do use but also for how much energy we do even have. But wait, isn’t that just a subsidy? Guess they have already found a way to tap in. 🙂
Tallbloke
I think that there is general agreement that separate radiative calculations for the troposphere are redundant.
The discussion on the barometric equations brings that out.
What is even more surprising is the same seems to be true for a moist or wet atmosphere
Click to access Moist_adiabatic_lapse_rate.pdf
All explained by classical thermodynamics.
So what has happened to the bogeyman CO2 in the atmosphere?
It is certainly true that its radiative properties are quite different to say N2 or O2.
The heat capacity of CO2 increases by 13% between 250K and 350K whereas N2 is almost constant in this temperature range.
G&T say that the radiative properties of a gas are inextricably linked to the other thermal properties of the gas.
In a heat transfer situation bulk properties like heat capacity include the radiative contribution.
Because the partial pressure of the trace gas CO2 in air is so small compared to N2 and O2 that even doubling CO2 will have a negligible effect on air .
However if the situation were reversed with say an 80% CO2 atmosphere then one of the assumptions of the barometric equations breaks down.
Namely that the heat capacity is independent of temperature.
It would seem to indicate that the real role of CO2 in above the troposphere where the radiative properties are highly significant which is very much in line with the top post.
tallbloke 2:56pm: “It isn’t acheived physically, only inside theoretical models.”
“…or there’s a ‘photon bath’ and it can’t.” Agree this one in nature, only the net bath is useful not the gross amount. Cannot extract gross UWIR or gross DWIR in the 2 stream approx. either, only net.
The top post picture is made entirely by theoretical net radiative transfer not gross.
The same picture is made by radiosonde data so is achieved physically in the real atmosphere both tropics and subarctic within instrument error.
The two stream model cannot be made to extract useful energy either from gross UWIR or DWIR (only the net) just like the more physical multiscattered bath cannot give up its energy for useful work without decreasing universe entropy. The two stream is simply a foundational tool, a step, on the hierarchy for building intuition of more complex multiscattering (like in a cloud). This is the power of the 2LOT; informed, critical folks have long since stopped searching for gross useful energy except in the net amount. There was an ongoing cottage industry searching in the 1800s trying to do so, 2LOT stopped it. As Tim F. writes 4:47pm only the net can be made useful. Relevant examples:
1) Solar in net of albedo is useful
2) Terrestrial UWIR net of terrestrial DWIR is useful in two stream approx. Advance study on more precise multiscattering does not over turn this finding – nor does it end the study as science is still practicing nature.
Bryan asks: “So what has happened to the bogeyman CO2 in the atmosphere?”
The main effect of CO2 is not its effect on heat capacity
The main effect of CO2 is not its effect on radiative transfer within the atmosphere.
The main effect of CO2 is not its effect on lapse rate.
The key effect is the effect on the radiation that escapes to space from the top of the atmosphere. Adding more cold CO2 at the top means less radiation escapes from the warmer CO2 lower down. This in turn mean more energy accumulating with in the atmosphere, which means warming within the atmosphere.
tallbloke says:
September 22, 2014 at 2:56 pm
The radiative greenhouse effect is tiny compared to the effect of gravity acting on atmospheric mass to generate higher pressure (and therefore density) near the surface.
I agree. Gravity gradient causes pressure gradient and density gradient
.
That more densely packed medium intercepts more solar energy
Incoming photons energise W.V. Which heat, by collision ( thermalize ) N2 and O2
and outgoing longwave
Outgoing photon also thermalize N2 and O2.
from the ocean which absorbs the bulk of the solar energy.
That’s why the surface air is warmer than air at altitude.
You have described what I call radiative LR. You seem not to like “radiative because it is also used to describe the un- physical back-radiation concept.
Not because the near surface atmosphere is heated by ‘downwelling longwave radiation ‘trapped’ higher in the atmosphere,
I also don’t accept this CO2 trapping and back radiating.
but because the radiation almost immediately thermalised in the dense near surface air
There are reasonable radiative effects such as “intercepts more solar energy”
and unreasonable radiative effects such as back radiation.
is slow to convect and pass evaporated water upwards due to its density.
This part of your explanation is not needed.
The top graphic shows W.V. cools, that is radiates energy to space from all levels. Not mainly from high up. It is colder there.
Convection and evaporation move molecular kinetic energy. Molecules that go up must come down. The falling air and water carry the energy back down.
There is the radiation cycle and the mass/heat cycle.
Bryan: It would seem to indicate that the real role of CO2 [is] above the troposphere where the radiative properties are highly significant
Yes, CO2’s real role is radiating to space, cooling the planet.
Tim F: The key effect is the effect on the radiation that escapes to space from the top of the atmosphere. Adding more cold CO2 at the top means less radiation escapes from the warmer CO2 lower down.
Tim is back to his old ‘throttling’ argument. We’ve been through this many times. Extra co2 at the top of the atmosphere does not ‘throttle’ heat throughput from below. Indeed OLR has been increasing over the last couple of decades as co2 has increased according to the satellite DATA.
Trick: informed, critical folks have long since stopped searching for gross useful energy except in the net amount.
The net flow is upwards, and therefore you agree that the greenhouse concept is a dead duck unless you are going with Tim’s throttling argument, which is contradicted by the DATA.
Tallbloke says: “ Indeed OLR has been increasing over the last couple of decades as co2 has increased according to the satellite DATA. “
Could you point me toward a source for this claim? It would be interesting to learn more about this.
tallbloke 9:44pm: The data show 288K at surface and data show 255K at satellite.
You check your post over and over and then you hit the “submit” button. Thirty seconds later you realize you made a dumb mistake.
What to do? Issue an “Ooops!” or wait to see if anyone spots your dumb error?
I posted this:
Cp = M * (γ – 1) / γ / R
Unfortunately the equation is wrong. Here is the correct version:
Cp = γ * R / M/ ( γ – 1)
The strange thing is that the error does not matter if you use the constants provided in Roger Clague’s link (http://physics.ohio-state.edu/~jay/846/more.pdf)
That bright spark from Ohio State with his elegant derivation of the DALR must be about my age; he learned his physics when “cgs” ruled. Like me he got a little confused when everything went “mks”. Thus he used the wrong value for the molecular weight of air (29 instead of 0.029):
M * (γ – 1) = 29 * 0.4 = 11.6
γ * R = 8.31 * 1.4 = 11.634
Thus according to Ohio State Cp = 1.0 so it made no difference that I got things upside down. The lapse rate came out at 9.8 K/km because g = 9.81 m/s2.
If the Ohio State guy had not confused his units Cp would have equaled 1,006 kJ/kg.K rather than 1.006 in Ohio State units
Tim Folkerts says: September 22, 2014 at 4:47 pm
(Tallbloke says: “If there really was a separate downwelling stream of 330W/m^2, we would be able to harness the energy, but we cannot.”)
“You don’t understand the 2nd Law if you make such a claim. Here are few brief arguments.”
The 2nd Law of thermodynamics addresses only the conditions of spontaneous heat transfer.
Higher temperature to lower temperature cannot be spontaneous, ever!
“1) Consider a similar situation — air molecules bouncing around in a room. At any given moment, about 1/2 of the molecules are heading upward and about 1/2 are heading downward. There really, truly are “downwelling molecules” and other “upwelling molecules” at any given moment. Do you think we should be able to harness the energy of only the downwelling molecules?”
You seem to confuse Newtonian kenetic energy, a vector value, with HEAT, the vectorless Poisson distribition of gas molecules. Try to harness that energy without a identifiable temperature/pressure difference.
“2) If I put a colder object on the surface (say a liquid nitrogen cooled plate), then you COULD harness the 330 W/m^s of downwelling IR. This suggests the photons are there all along, but that the greater upwelling flow of photons prevents the effective use of these photons.”
You cannot any thermal flux in a direction of higher radiance. That introduction of a lower radiance surface allows detachment of EMR flux from a higher radiance surface.
“3) You also cannot harness the ~ 390 W/m^2 of downwelling IR — at least not all of it. Only the NET flow can be harnessed. And even more, the net flow is limited by the temperature differences.”
I think you ment the ClimAstrology claimed upwelling. Nomatter, try to harness any thermal EMR flux/flow between isotherms
“hmmm … that might be a good challenge for people to contemplate. A 1 square meter 300 K object radiating to 282 K surroundings loses power at a rate of 100 W/m^2. So does a 250 K object radiating to 215 K surroundings. Or a 220 K object radiating to 155 K surroundings. But in the first case, we can only extract work at a rate of up to 6 W. For the second we could extract up to 14 W. For the last we could extract up 29 W. The amount of work that can be extracted depends not on the NET flow, but on the sizes of the upward and downward flows!”
What total nonsense!! In each case the higher temperature surface must be supplied with 100 watts of power, likewise the lower temperature surface must dissapate 100 watts of power to yet colder, just to maintain your given isotherms. Please show how any of this EMR power can somehow be “extracted”, and in what form?
“(In the end it doesn’t really matter. Proper application of either the opposing photon streams or net flux will lead to the correct answers.)”
The Schuster-Schwarzschild two stream approximation can “never be properly applied” to a dissipative atmosphere. On This Earth you do not even have your so called net flux.
Trick says: September 22, 2014 at 10:53 pm
“tallbloke 9:44pm: The data show 288K at surface and data show 255K at satellite.”
What satellite measures temperature? Temperature of what? Where? What is temperature supposed to indicate?
Tim Folkerts says: September 22, 2014 at 8:38 pm
“The key effect is the effect on the radiation that escapes to space from the top of the atmosphere. Adding more cold CO2 at the top means less radiation escapes from the warmer CO2 lower down. This in turn mean more energy accumulating with in the atmosphere, which means warming within the atmosphere.”
Show any increase in temperature within the atmosphere from higher CO2 amounts.
Adding atmospheric CO2 can only increase EMR exitance from the atmosphere. This is sasillyn compensated “if wished” by slightly less atmospheric H2O.
Tim F: Could you point me toward a source for this claim? It would be interesting to learn more about this.
Siberia


Canada


Atlantic


A curiosity:
http://faculty.washington.edu/kessler/ENSO/clouds-n-olr.html
So more cloud – less OLR.
But also less insolation to surface
Reed (1977) found the empirical formula Q=Q0(1-0.62C+0.0019alpha), where C is total cloud cover and alpha is noon solar angle. (For C less than 0.3, Reed finds no reduction in insolation (Q = Q0)).
Trick: The data show 288K at surface and data show 255K at satellite.
Yes, amazing what gravity does acting on the mass of the atmosphere isn’t it?
Will Janoschka says, September 23, 2014 at 8:06 am:
“Trick says: September 22, 2014 at 10:53 pm
“tallbloke 9:44pm: The data show 288K at surface and data show 255K at satellite.”
What satellite measures temperature? Temperature of what? Where? What is temperature supposed to indicate?”
Exactly. What is measured (well, estimated from a bunch of different measurements) is the global mean surface temperature AND an average radiative flux to space from the Earth system as a whole of 240 W/m^2. No ‘Earth system temperature’ is measured.
So why is Earth emitting an average radiative flux to space at 240 W/m^2? Because that is the average flux it happens to absorb from the Sun (evened out over the global surface and across the diurnal cycle). There is nothing more to it. The mean temperature of Earth’s global solar-heated surface is 288K because there is a massive (warm & heavy) atmosphere resting on top of it, insulating it by setting a limit to its convective/evaporative heat loss at a certain temperature.
It’s all so plain and simple.
tallbloke says: September 23, 2014 at 8:52 am
“Yes, amazing what gravity does acting on the mass of the atmosphere isn’t it?”
The weight (=surface pressure) of the atmosphere is equal to that of ~10 meter of water.
If the pressure of the atmosphere can increase the temperature of the surface some 80 or 90K above that of the moon, why isn’t the temperature increasing with increasing depth of the oceans?
Pressure at 4km deep is about 400 bar. Should give an enormous temperature rise following this logic.
Ben, no, because water isn’t compressible to anything like the degree air is. Higher relative near surface air density is what underlies the lapse rate.
Ben Wouters says: September 23, 2014 at 9:55 am
“The weight (=surface pressure) of the atmosphere is equal to that of ~10 meter of water.
If the pressure of the atmosphere can increase the temperature of the surface some 80 or 90K above that of the moon, why isn’t the temperature increasing with increasing depth of the oceans?
Pressure at 4km deep is about 400 bar. Should give an enormous temperature rise following this logic.”
Why do you think the surface temperature of the Earth and Moon should be related?
What is the increase in density of the liquid water at a depth of 4000 meters?
Will Janoschka says:
September 23, 2014 at 7:51 am
The Schuster-Schwarzschild two stream approximation can “never be properly applied” to a dissipative atmosphere.
There is a difference of opinion as to how best to understand the atmos.
1. Ideal Gas Laws (IGL) for molecules
2. Two stream approximation (TSA) for photons
As a chemist I revere the IGL. However I have to accept its limit, the lab and the chemical factory.
1. IGL
requires:
(a) constant g g varies by 3/1000
(b) homogeneous T T varies by 1/4
(c) T near STP
(d) P near STP
(c) a container with solid walls in all directions
2.TSA
Was developed Schwarzwald (1906) to understand the sun and other stars. Manabe ans Strickler (1964) applied to the Earth.
Assumptions
(1)The total radiation energy absorbed is from the sun above and the surface below.
(2)Scattering can be ignored because energy scattered out equals energy scattered in
(3)The temperature at any height is due to the total radiation absorbed.
None of the IGL condition are needed. TSA was developed because IGL could not be used in stars.
This method has been successfully applied to many planetary atmos.:
Click to access Robinson2014_0.1bar_Tropopause.pdf
Kristian says: September 23, 2014 at 9:41 am
“So why is Earth emitting an average radiative flux to space at 240 W/m^2?”
Just who and what has ever measured total EMR exitance from the Earth system?
How could that possibly done without observing the whole disk of the Earth system?
“Because that is the average flux it happens to absorb from the Sun (evened out over the global surface and across the diurnal cycle).”
Just who and what has ever measured total Solar EMR flux absorbed by the Earth system?
Is EMR flux the only way the Earth system receives energy?
“The mean temperature of Earth’s global solar-heated surface is 288K because there is a massive (warm & heavy) atmosphere resting on top of it”,
OK, Why is it not some other temperature?
“insulating it by setting a limit to its convective/evaporative heat loss at a certain temperature.””
What part of the atmosphere does this insulating? How is convective/evaporative heat transfer limited?
“It’s all so plain and simple.”
Only if you think you know “how” this planet works!
All of your claims are those of ClimAstrologists who think they know!
What is temperature supposed to indicate?”
What is “mean temperature” supposed to indicate?”
Will Janoschka says: September 23, 2014 at 10:25 am
“Why do you think the surface temperature of the Earth and Moon should be related?”
Perhaps because they circle the same sun at the same distance (give or take a few kilometres)?
Although earth reflects away more solar than the moon (~30% vs ~11%) the earth has a much higher temperature than the moon.
“What is the increase in density of the liquid water at a depth of 4000 meters?”
I’m aware that water is hardly compressible.
I’m trying to get the idea across that it is nonsense to look at the atmosphere with a heat capacity equal to ~3 meter of water and a weight equal to ~10 meters of water to explain our exceptionally high temperatures. The atmosphere or the sun are unable to warm the deep oceans to any appreciable depth. Geothermal obviously is.
Roger Clague says: September 23, 2014 at 10:53 am
Will Janoschka says: September 23, 2014 at 7:51 am
(“The Schuster-Schwarzschild two stream approximation can “never be properly applied” to a dissipative atmosphere.”)
“2.TSA
Was developed Schwarzwald (1906) to understand the sun and other stars. Manabe and Strickler (1964) applied to the Earth.”
Schwartzwald was a poet! Manabe-Strickler has interesting graphs but is not any two stream approximation. It is also incorrect.
“Assumptions
(1)The total radiation energy absorbed is from the sun above and the surface below.”
That assumption is ridiculous, surface radiation is limited by the immediate opposing radiance. The atmosphere not the surface radiates.
“(2)Scattering can be ignored because energy scattered out equals energy scattered in”
What kind of scattering is equal?
“(3)The temperature at any height is due to the total radiation absorbed.”
Not on this planet! Temperature mainly via WV condensation vs radiative exitance of the atmosphere at that height.
“None of the IGL condition are needed. TSA was developed because IGL could not be used in stars.”
True, The SSTSA applies only to luminious stars
“This method has been successfully applied to many planetary atmos.:”
Name even one sucessfull application of SSTSA to a planet?
“http://faculty.washington.edu/dcatling/Robinson2014_0.1bar_Tropopause.pdf”
The Robinson-Catling paper is about a 0.1 bar pressure at the lowest cold inflection nothing to do with any two stream approximation. 0.1 bar is the pressure in a gravitational field where changes in degrees of freedom for a gas begin.
The Schuster-Schwarzschild two stream approximation can “never be properly applied” to a dissipative atmosphere.
Ben Wouters says: September 23, 2014 at 12:37 pm
“I’m trying to get the idea across that it is nonsense to look at the atmosphere with a heat capacity equal to ~3 meter of water and a weight equal to ~10 meters of water to explain our exceptionally high temperatures. The atmosphere or the sun are unable to warm the deep oceans to any appreciable depth. Geothermal obviously is.”
The surface pressure (not weight) is equal to the pressure of a 32 foot open column of water near the surface. The weight of the atmosphere is the same as 30 inches of mercury covering the whole earth surface. There is no exceptionally high temperatures! Adjustable atmospheric WV keeps surface temperature correct. Is there any meaning to “a heat capacity equal to ~3 meter of water? Any length of water with no mass has no heat capacity. Please state the correct latent heat of all WV still in the atmosphere?
Ben W: The atmosphere or the sun are unable to warm the deep oceans to any appreciable depth. Geothermal obviously is.
Well I don’t think we can be sure of any of that. It’s plausible, but so is the possibility that the overturnings of the oceans by lunar tidal actions and LOD changes are sufficient to keep the depths at the temperature they are (with some help from geothermal).
Rule nothing out.
Tallbloke,
I am not surprised that the OLR is strongly correlated to cloud cover (ie to absorbed incoming shortwave radiation).
But consider a more subtle relationship, discussed here: https://www.eumetsat.int/cs/idcplg?IdcService=GET_FILE&dDocName=pdf_conf_p50_s9_01_harries_v&allowInterrupt=1&noSaveAs=1&RevisionSelectionMethod=LatestReleased
This shows the OLR across the IR spectrum. In particular, the last graph shows the difference between data from 1970 to 2006. The overall IR is pretty steady (maybe a little higher in 2006). But within the absorption bands for CO2 and O3 and CH4, there is LESS OLR. These GHGs are blocking more IR within the bands where they are effective absorbers. This pushes more IR to other parts of the IR spectrum — parts that come from the ground or lower clouds.
So we have TWO separate effects:
* clouds cause an overall change in ISR and thus an overall change in OLW (ie more clouds = cooler = less OLR).
* GHGs cause specific decreases in OLR at some wavelengths (ie weak IR from the TOA), which is compensated by increased OLR at other wavelengths (ie through the atmospheric window from the warmer surface).
One does not negate the other.
Will Janoschka says: September 23, 2014 at 1:40 pm
“There is no exceptionally high temperatures!”
Avg. surface temperature of earth ~290K, the moon ~197K.
Seems to me earth is a lot warmer than the moon, although earth reflects away more solar energy than the moon. How do you explain this difference?
“Adjustable atmospheric WV keeps surface temperature correct.”
How does WV keep the temperatures on earth more than 90K above those on the moon?
Tim, OK, that kind of fits the obs. OLR in the northern Hemisphere has been increasing since the early 80s, despite co2 emissions and airborne levels having accelerated. This is due to diminishing cloud amount 1980-1998 (observed by ISCCP) letting more Sun in, and consequently more energy being shed as the Earth maintains its near-homeostatic balance. Less cloud means more OLR from the surface and less need for CO2 to get involved. ‘Excess’ CO2 is along for the ride. Surplus to requirements. Redundant. Didn’t prevent OLR from leaving in increasing amounts as temperature rose. Negative feedback wins. No ‘throttling’ effect.
tallbloke says: September 23, 2014 at 1:53 pm
“It’s plausible, but so is the possibility that the overturnings of the oceans by lunar tidal actions and LOD changes are sufficient to keep the depths at the temperature they are (with some help from geothermal).”
How did lunar tidal action and LOD changes heat the deep oceans in the Cretaceous to some 18K above present temperatures?
Will Janoschka says: September 23, 2014 at 1:40 pm
“Is there any meaning to “a heat capacity equal to ~3 meter of water?”
Perhaps you understand this easier:
The heat capacity of the total atmosphere is about the same as that of the top 3 meters of the oceans.
Ben W: How did lunar tidal action and LOD changes heat the deep oceans in the Cretaceous to some 18K above present temperatures?
How do you know whether the deep ocean before the Cretaceous was warmer or cooler?
Ben Wouters says: September 23, 2014 at 2:12 pm
Will Janoschka says: September 23, 2014 at 1:40 pm
(“There is no exceptionally high temperatures!”)
“Avg. surface temperature of earth ~290K, the moon ~197K.”
OK Average surface temperature has no meaning!
“Seems to me earth is a lot warmer than the moon, although earth reflects away more solar energy than the moon. How do you explain this difference?”
The Earth receives energy from other than EMR from the Sun perhaps. Moon has no magnetic field of its own. The two bodies are nowhere similar!
(“Adjustable atmospheric WV keeps surface temperature correct.”)
“How does WV keep the temperatures on earth more than 90K above those on the moon?”
I don’t know! Are you claiming the Earth’s surface temperature is incorrect?
Ben Wouters says: September 23, 2014 at 2:48 pm
Will Janoschka says: September 23, 2014 at 1:40 pm
(“Is there any meaning to “a heat capacity equal to ~3 meter of water?”)
“Perhaps you understand this easier:”
“The heat capacity of the total atmosphere is about the same as that of the top 3 meters of the oceans.”
What does the difference in specific heat between air and water have to do with anything?
The temperature of the whole atmosphere changes much, the top 3 meters of ocean not much. Please state the correct latent heat of all WV still in the atmosphere, as that is the real heat capacity of the atmosphere in Joules?
tallbloke says: September 23, 2014 at 3:04 pm
“How do you know whether the deep ocean before the Cretaceous was warmer or cooler?”
See http://commons.wikimedia.org/wiki/File:Phanerozoic_Climate_Change.png
Around 110 mya earth came out of a cold glacial period. Is incompatible with hot oceans.
Ben Wouters says:
September 23, 2014 at 9:55 am
If the pressure of the atmosphere can increase the temperature of the surface some 80 or 90K above that of the moon, why isn’t the temperature increasing with increasing depth of the oceans?
Pressure at 4km deep is about 400 bar. Should give an enormous temperature rise following this logic. and later
I’m trying to get the idea across that it is nonsense to look at the atmosphere with a heat capacity equal to ~3 meter of water and a weight equal to ~10 meters of water to explain our exceptionally high temperatures. The atmosphere or the sun are unable to warm the deep oceans to any appreciable depth. Geothermal obviously is.
Good question
You say:
1 bar atmos (10km ) causes dT = 90K
1 bar ocean (10m ) causes dT = 0K should also cause 90K.
I don’t calculate it that way. I say:
change in ave velocity of molecules (dV) causes dT (K.E. theory, not IGL)
Atmos.
dV= change in gravity x distance applied
= 0.003 x 10 000m
= 300ms^2
Ocean
change in gravity = (6000.01km)^2/ (6000km)^2 xg
= 360000120/36000000 xg
= 3x 10^-7 x 10ms^-2
= 3x 10^-6ms^-2
dV = 3x 10^-6ms^-2 x 10m
= 3x 10^-5ms^-1
That the same weight of ocean as the atmos has a negligible efect on the temperature of that ocean.
Will Janoschka says, September 23, 2014 at 11:42 am:
“Just who and what has ever measured total EMR exitance from the Earth system?
How could that possibly done without observing the whole disk of the Earth system?”
You have a satellite or two with IR-detecting instruments (like CERES on the Terra & Aqua satellites). They circle the Earth, measuring IR flux from Earth as they move. They make thousands of different measurements, from the poles to the equator, from the eastern to the western hemisphere. They do this for several years. Endless rounds. You end up with a mean value. This mean value closely matches the evened out incoming flux from the Sun. No wonder. They should and must balance.
“Just who and what has ever measured total Solar EMR flux absorbed by the Earth system?
Is EMR flux the only way the Earth system receives energy?”
Several satellites detect TSI from the Sun. To spread this flux evenly across the globe and the diurnal cycle (or over a year, for that matter), you divide it by 4. Then you have to figure out Earth’s average global albedo. This you also do by satellite sensors measuring reflected radiation (SW mostly). You do it by taking thousands upon thousands of individual measurements, and then finally, after a few years, you have a mean value. For Earth in space this is about 0.3. So you time the TSI diveded by 4 with 0.7. And what do you get? 238-240 W/m^2. Exactly the same as the average outgoing IR flux from Earth to space. Lo and behold, they match! Balance. Did anyone expect otherwise? Nature’s fine-tuned machinery!
I find this whole thing not a very controversial topic. It’s fairly straightforward. But you apparently do. I guess this is also all a part of the grand nuevo science/climastrology scam, isn’t it, Will?
“OK, Why is it not some other temperature?”
What kind of question is that? Because it isn’t. That’s the temperature it needs to be at. To be able to balance incoming with outgoing. Apparently. With our current input from the Sun (included our current global albedo). And with the current mass (weight, heat capacity) of our atmosphere.
“What part of the atmosphere does this insulating? How is convective/evaporative heat transfer limited?”
Again a weird question to ask. What part? All of it. The atmosphere AS A WHOLE, as a thing, does the insulation. No particular PART of it. The fact that it has a mass.
Convective/evaporative heat loss for the surface is limited at a certain temperature level because 1) the atmosphere, having a mass, has a ‘heat capacity’, hence it is able to warm; it primarily warms convectively from the surface; it therefore sets up a certain temperature gradient away from the solar-heated surface; as you know, in convective transfer, the steepness of temperature gradients matter; and 2) the atmosphere, having a mass, also has a ‘weight’ and a density; this leads to two effects – a) the air gets ‘heavy’, which affects the buoyant acceleration (upward momentum) of air at a certain temperature, and b) the atmosphere itself presses down on the surface with a certain total weight (downward force), expressed by surface air pressure, this affects evaporation rates from especially the ocean at a certain surface temperature, which again affects buoyant uplift of air.
The atmosphere in principle insulates the solar-heated surface of the Earth in the same manner as everyday insulation does (like clothes, blankets and walls/ceilings), only not with a rigid blockage of air movement, rather with a restriction of free (ideal) movement.
““It’s all so plain and simple.”
Only if you think you know “how” this planet works!
All of your claims are those of ClimAstrologists who think they know!”
Yeah, that’s rich. I’m such a Climastrology fanboy. I swallow everything they serve me. You just keep on believing that, Will …
Will asks: “Just who and what has ever measured total EMR exitance from the Earth system?”
See the graphs Tallbloke posted. Several satellites measure earth’s IR.
“How could that possibly done without observing the whole disk of the Earth system?”
Satellites *do* measure the whole disk over the course of several orbits. Combining the results at various times and places gives the total.
“Just who and what has ever measured total Solar EMR flux absorbed by the Earth system?”
Same answer. Satellites routinely measure insolation and albedo. (And there are various other ways to measure albedo as well). From these it is trivial to calculate the total power absorbed
“Is EMR flux the only way the Earth system receives energy?”
Yes, pretty much.
* The sun averages ~ 340 W/m^2 of incoming power (with ~ 100 W/m^2 of that reflected away).
* The full moon is on the order of 0.001 W/m^2
* Geothermal is estimated to be ~ 0.1 W/m^2 up from the interior.
* I suppose you could consider solar wind & infalling meteors, but that would be even smaller.
* Some moons of Jupiter have significant tidal heating, but that would not apply to the earth.
If you have another mechanism to propose, tell us what it is. Otherwise, it is clear that over 99% of the received energy comes from the sun, so other sources can be pretty much ignored.
tallbloke 8:52am: Concur.
Gravity’s effect acting on the incremental optical depth d(tau) of an atmosphere mass enters the top post picture as shown in the Robinson & Catling 2013 paper eqn. S15 where also factoring in mass extinction effects (grey opacity) of “various absorbing gases” computes out to the difference for Earth global surface measured 288K and satellite measured 255K as shown in the paper.
Same picture as top post could be constructed from line by line radiative transfer alone for Venus (et. al. planets/moon with thick atmospheres) on which gravity acts as shown in R&C13 paper, leading to the even more amazing effect of gravity shown in difference of Venus surface sparsely measured around 732K and satellite measured around 232K.
******
Will 8:06am: ?s
All satellites and rovers currently measure temperature in order and in part to report their health.
Satellites such as Earth Radiation Budget Experiment (ERBE) and the Clouds and the Earth’s Radiant Energy System (CERES) measure the brightness temperature of an earth scene looking down or a space scene looking up using radiometers.
Trick: You are not concurring with me at all 😉
Correction to
September 23, 2014 at 7:46 pm
Atmos
change in Velocity = 0.003x 10m/s2 x 10 000m
= 300m/s
tallbloke 9:41pm: Not concurring? Please explain. Gravity is amazing in what it does acting on the mass of the atmosphere. So is the top post picture amazingly in accord with test. R&C13 detail it.
@Roger Clague, September 23, 2014 at 7:46 pm
“Ben Wouters says:
September 23, 2014 at 9:55 am
If the pressure of the atmosphere can increase the temperature of the surface some 80 or 90K above that of the moon, why isn’t the temperature increasing with increasing depth of the oceans?
Pressure at 4km deep is about 400 bar. Should give an enormous temperature rise following this logic.”
The theoretical lapse rate in the atmosphere is negative (-g/Cp) as shown by Rodrigo Caballero and (elegantly) by a geezer at Ohio State.
The lapse rate for the oceans is positive so temperature falls with depth until water approaches its maximum density at a temperature ~276 K. This is hardly news. In 1866 William Thompson (Lord Kelvin) was a consultant to Cyrus Field who wanted to a lay a trans-Atlantic telegraph cable. Thompson supervised the testing of the 6,000 tonnes of cable which was installed in 14 huge tanks in Greenwich (UK). The tanks were flooded with water maintained at a temperature of 4 degrees Centigrade appoximating the average temperature the cable would be exposed to at depths of up to 4,000 meters.
Why is the lapse rate in water positive? Simply because water expands as temperature rises and this effect overwhelms the contraction due to pressure.
gallopingcamel says: September 24, 2014 at 1:31 am
“Why is the lapse rate in water positive? Simply because water expands as temperature rises and this effect overwhelms the contraction due to pressure.”
I find most earthlings cannot walk and chew gum at the same time. Fine responce!
Water has many properties that other gasses liquids and solids don’t. Water exists in all three phases under conditions typical on Earth. The heat contents of the three phases are vastly different, yet it is possible to find all three phases together at the same temperature.
At atmospheric pressure water is at its maximum density at about 4 deg. C . Water is very slightly compressible, and its enthalpy increases as it is compressed, Water at pressure greater than about 221 bar is supercritical, and the phase distinction between liquid and vapor ceases to exist. In the oceans, this occurs at the critical depth of about 1.4 mi, or 2.2 km. Ocean temperature at this depth and below appears to remain nearly constant. My guess is that it is maintained at its maximum density temperature by the pressure, i.e. the heat is literally squeezed out of it.
I also suspect that geothermal heat entering the bottom is relayed as a pressure wave to become vapor above the critical depth. As the vapor forms it is at only a slightly lower density, so it rises. As it rises, it also increases in volume. Some of the vapor mixes with surface waters and condenses and some pierces the surface and rises in the atmosphere as water vapor,
.
On all scales but volume, water overwhelms air. The oceans are like a keg of beer. Ocean surface waters are like a draft in an open mug. The atmosphere is like its head. The land is like the mug. .
I’ve never met most earthlings.
Stops to ponder, moves on falls flat on face. Dang gum on broadwalk.
Kristian says: September 23, 2014 at 8:12 pm
Will Janoschka says, September 23, 2014 at 11:42 am:
(“Just who and what has ever measured total EMR exitance from the Earth system?
How could that possibly done without observing the whole disk of the Earth system?”)
“You have a satellite or two with IR-detecting instruments (like CERES on the Terra & Aqua satellites). They circle the Earth, measuring IR flux from Earth as they move. They make thousands of different measurements, from the poles to the equator, from the eastern to the western hemisphere. They do this for several years. Endless rounds. You end up with a mean value. This mean value closely matches the evened out incoming flux from the Sun. No wonder. They should and must balance.”
Aqua was the only satellite that measured radiance other than from nadir and all of them have a fixed FOV (steradiance). None can possibly measure the total radiand intensity from the Earth. Only a WFOV radiometer no closer than the Moon could possibly measure such! You have been fed more BS from NASA.
(“Just who and what has ever measured total Solar EMR flux absorbed by the Earth system?
Is EMR flux the only way the Earth system receives energy?”)
“Several satellites detect TSI from the Sun. To spread this flux evenly across the globe and the diurnal cycle (or over a year, for that matter), you divide it by 4.”
That is irradiance, never flux, That irradiance must be decreased by any opposing radiance from the Earth. Since most Solar irradiance is less than 2.5 microns there is little opposing radiance. That incident flux is never distributed evenly across the earths surface Solar flux at the terminator must be zero W/m2.
“Then you have to figure out Earth’s average global albedo. This you also do by satellite sensors measuring reflected radiation (SW mostly). You do it by taking thousands upon thousands of individual measurements, and then finally, after a few years, you have a mean value.”
Albedo is defined as visable backscatter from the disk of a body illuminated by its primary. It does not include any backscatter from less than 0.4 microns or more than 0.7 microns. It also does not include any of the considerable forward scattering from the atmosphere at any wavelength.
Your TSI (1-0.3)/4 is part of the BS promoted by ClimAstrologists! When will you ever learn.
“For Earth in space this is about 0.3. So you time the TSI diveded by 4 with 0.7. And what do you get? 238-240 W/m^2.”
1340 W/m^2 (TSI) x 0.5 (acceptance of atmosphere and surface) is confined to surface normals +/- 45 degrees another 0.5 of the cross-sectional area of the earth. Please show any “measurement”, not fake calculation of nonsense, that has ever been made?
“Exactly the same as the average outgoing IR flux from Earth to space. Lo and behold, they match! Balance. Did anyone expect otherwise? Nature’s fine-tuned machinery!”
Your favorite ClimAstrologists claim is 0.6 W/m^2 more in than out, CAGW, Yet you use only the ClimAstrology claims to show their claims are incorrect!
“I find this whole thing not a very controversial topic. It’s fairly straightforward. But you apparently do. I guess this is also all a part of the grand nuevo science/climastrology scam, isn’t it, Will?)
Yes it is. The controversy is why you keep eating the garbage they serve up, when you know it is garbage. Why not try to think if” why” it is continuing garbage?
(“OK, Why is it not some other temperature?”)
“What kind of question is that? Because it isn’t. That’s the temperature it needs to be at. To be able to balance incoming with outgoing. Apparently. With our current input from the Sun (included our current global albedo). And with the current mass (weight, heat capacity) of our atmosphere.”
And the variable WV in the atmosphere plays no part I suppose! What controls the current amount of atmospheric WV? Why does the Specific heat of the atmosphere have anything to do with atmospheric or surface temperature, with latent heat and radiative exitance from the atmosphere? To be continued
Kristian says: September 23, 2014 at 8:12 pm
Will Janoschka says, September 23, 2014 at 11:42 am:
Continued
(“What part of the atmosphere does this insulating? How is convective/evaporative heat transfer limited?”)
(“Again a weird question to ask. What part? All of it. The atmosphere AS A WHOLE, as a thing, does the insulation. No particular PART of it. The fact that it has a mass.”)
The WV in the atmosphere and the emissivity of that atmospheric WV and clouds provide
a more efficient way of radiating energy to space than the surface can do!
“Convective/evaporative heat loss for the surface is limited at a certain temperature level because 1) the atmosphere, having a mass, has a ‘heat capacity’, hence it is able to warm; it primarily warms convectively from the surface; it therefore sets up a certain temperature gradient away from the solar-heated surface; as you know, in convective transfer, the steepness of temperature gradients matter; and 2) the atmosphere, having a mass, also has a ‘weight’ and a density; this leads to two effects – a) the air gets ‘heavy’, which affects the buoyant acceleration (upward momentum) of air at a certain temperature, and b) the atmosphere itself presses down on the surface with a certain total weight (downward force), expressed by surface air pressure, this affects evaporation rates from especially the ocean at a certain surface temperature, which again affects buoyant uplift of air.”
Any increase in surface pressure is more than compensated by the increase in WV partial pressure do to that same increase in temperature.
“The atmosphere in principle insulates the solar-heated surface of the Earth in the same manner as everyday insulation does (like clothes, blankets and walls/ceilings), only not with a rigid blockage of air movement, rather with a restriction of free (ideal) movement. It’s all so plain and simple.”
There is no restriction especially with the great lateral winds providing increased evaporation and a fine motor for your seacraft.
(“Only if you think you know “how” this planet works!
All of your claims are those of ClimAstrologists who think they know!”)
“Yeah, that’s rich. I’m such a Climastrology fanboy. I swallow everything they serve me. You just keep on believing that, Will …”
Your own written words demonstrate that you keep on swallowing! Not one word, phrase, sentence, paragraph, chapter, or article is in any way correct from ClimAstrologists, even the punctuation is suspect.
[mod: cool it you two. –Tim]
tchannon says: September 24, 2014 at 4:15 am
“I’ve never met most earthlings. Stops to ponder, moves on falls flat on face. Dang gum on broadwalk.”
OK sorry, Most earthlings that I have had the misfortune to observe, walking, chewing!
Mysterious verb precedents intentional.
Tim Folkerts says: September 23, 2014 at 8:33 pm
(Will asks: “Just who and what has ever measured total EMR exitance from the Earth system?”)
“See the graphs Tallbloke posted. Several satellites measure earth’s IR.”
They only measure “radiance” from a paticular direction with a very limited FOV.
“(How could that possibly done without observing the whole disk of the Earth system?”)
“Satellites *do* measure the whole disk over the course of several orbits. Combining the results at various times and places gives the total.”
There can be no correct combining of those results. At the distance of the Moon the radiant intensity of the Earth can be and has been measured!
(“Just who and what has ever measured total Solar EMR flux absorbed by the Earth system?”)
“Same answer. Satellites routinely measure insolation and albedo. (And there are various other ways to measure albedo as well). From these it is trivial to calculate the total power absorbed”
See my response to Kristian on your rediculous claim of calculation, not measurement.
(“Is EMR flux the only way the Earth system receives energy?”)
“Yes, pretty much.
* The sun averages ~ 340 W/m^2 of incoming power (with ~ 100 W/m^2 of that reflected away).
* The full moon is on the order of 0.001 W/m^2
* Geothermal is estimated to be ~ 0.1 W/m^2 up from the interior.
* I suppose you could consider solar wind & infalling meteors, but that would be even smaller.
* Some moons of Jupiter have significant tidal heating, but that would not apply to the earth.”
That is what your ClimAstrologists have assumed!
Has the earth significant Lunar tidal energy increase? Why is the Moon so cold?
The Solar radiative bulge provides how much energy? Why?
Please calculate energy transfer for:
1) Charged and neutral particle flux.
2) Additional EUV, and gamma from CME’s.
3) Changes in rotational inertia of the earth.
4) Electromotive forces.
5) Magnetomotive forces.
Why does an induction motor in a dark basement increase in temperature when doing work?
Why defend the indefensible? Nobody knows!
Kristian says:
September 23, 2014 at 8:12 pm
Convective/evaporative heat loss for the surface is limited at a certain temperature level because 1) the atmosphere, having a mass, has a ‘heat capacity’, hence it is able to warm; it primarily warms convectively from the surface; it therefore sets up a certain temperature gradient away from the solar-heated surface; as you know, in convective transfer, the steepness of temperature gradients matter;
1. Normal convection
The solar heated surface,s,( T(s) =290K ) and the cooler tropopause,t,( T(t) = 220K ) cause the atmos.T gradient ( LR ).
LR = 290K -220K/12km.
= 6K/km
No need for c,heat capacity
no gradient of g/p/rho/weight
Similar to normal conduction/convection as in a room.
The difference in T from top to bottom causes the convection
LR causes convection
2. the atmosphere, having a mass, also has a ‘weight’ and a density; this leads to two effects – a) the air gets ‘heavy’, which affects the buoyant acceleration (upward momentum) of air at a certain temperature,
2. Adiabatic Expansion
Now we have:
a gradient of g/p/rho/weight
The hydrostatic equilibrium
This cause buoyancy and upward movement, that is convection
This convection causes adiabatic cooling
Convection causes cooler top, which causes T gradient ( LR)
Convection causes LR
I have rewritten your Theories 1 and 2(a) as I understand them.
They appear to me to be different and inconsistent.
b) the atmosphere itself presses down on the surface with a certain total weight (downward force), expressed by surface air pressure, this affects evaporation rates from especially the ocean at a certain surface temperature, which again affects buoyant uplift of air.
The relationship between surface air pressure evaporation rates is explained using vapour pressure theory.
The atmosphere in principle insulates the solar-heated surface of the Earth in the same manner as everyday insulation does (like clothes, blankets and walls/ceilings), only not with a rigid blockage of air movement, rather with a restriction of free (ideal) movement.
This says the atmosphere warms by restriction of movement
The previous paragraph says the atmosphere warms by convection
Both cannot be true.
Roger Clague says: September 24, 2014 at 10:46 am
Kristian says:September 23, 2014 at 8:12 pm
Convective/evaporative heat loss for the surface is limited at a certain temperature level because 1) the atmosphere, having a mass, has a ‘heat capacity’, hence it is able to warm; it primarily warms convectively from the surface; it therefore sets up a certain temperature gradient away from the solar-heated surface; as you know, in convective transfer, the steepness of temperature gradients matter;
1. Normal convection
The solar heated surface,s,( T(s) =290K ) and the cooler tropopause,t,( T(t) = 220K ) cause the atmos.T gradient ( LR ).
LR = 290K -220K/12km.
= 6K/km
No need for c,heat capacity
no gradient of g/p/rho/weight
Similar to normal conduction/convection as in a room.
———————————————————————————————————
Roger,
Nice answer, Can we get back to interesting weather, tornadoes, hurricanes and innocently ask “how dey do dat?
Roger Clague says: September 23, 2014 at 7:46 pm
I did hope it would be clear my question was kind of rhetorical.
I do not see how our measly atmosphere can INCREASE the temperature of especially the (deep) oceans. I DO see that the atmosphere is capable of reducing the speed of energy loss to space.
gallopingcamel says: September 24, 2014 at 1:31 am
“The theoretical lapse rate in the atmosphere is negative (-g/Cp) as shown by Rodrigo Caballero and (elegantly) by a geezer at Ohio State.”
g/Cp says NOTHING about the temperature profile of the STATIC atmosphere.
Although they’re a bit sloppy in their language sometimes, this should be clear in both texts.
The Ohio guy has this:
“Incidentally, meteorologists refer to this as the dry adiabatic lapse rate. The chief reason for this is the neglect of water vapor condensation (releases latent heat and raises the temperature) This condensation is also the reason for cloud caps on mountains and chinook and foehn winds.”
Makes it loud and clear he has not understood the Foehn effect at all.
Foehn (and Chinook) winds can only occur when rain ( or snow) falls from the upslope flowing air.
Is actually a nice demonstration of the DALR and SALR, often used in meteorological exams.
Roger Clague says: September 24, 2014 at 10:46 am
” The difference in T from top to bottom causes the convection
LR causes convection”
NO. Convection is caused by the pressure gradient force on a parcel of air which has a lower density than the surrounding air. It will continue to rise as long as its density is lower than that of the air it rises into. The rising parcel cools according either the DALR, or the SALR when condensation occurs.
The temperature profile of the air the parcel rises into decides how high the parcel will be able to rise.
@ gallopingcamel
All the sources that I checked concur that a POSITIVE lapse rate mean DECREASING temperature as you go up. So while most physicists would call this a “negative gradient”, meteorologist use the opposite sign convention. An inversion has a negative lapse rate.
Using the “standard” meteorological definition, the theoretical lapse rate in the atmosphere is POSITIVE, not negative as you have said a few times.
Tim, that’s why all of “your sources” have held you upside down of all of these years. 🙂
All of my sources, like the documentation papers creating the standard atmospheres, claim lapse rate is the change in temperature from a reference altitude, normally the surface at 0km and the ELR is negative. They list the lower troposphere lapse rate as -6.5 K/km, getting cooler as altitude increases. So you seem to want everyone now to speak inverted but I might ask: What is ‘your’ reference altitude then? Must be somewhere in space?
See:
http://www.astrohandbook.com/ch09/standard_atmos_1976.pdf
or
http://www.adac.aero/linked/us_standard_atmosphere_rev1.pdf (English units)
All of mine say the ELR is negative, of course DALR too.
Isn’t this a bit picking on gallopingcamel when he is technically correct? If you say 6.5 K/km I think most fellow commenters here know it is really -6.5 K/km. However this little difference does add confusion when someone says something like ‘when the lapse is under…’ or ‘when the lapse is exceeded…’, well, are they using the positive or negative sense? It then does matter to understand what is being said.
Wayne, I wasn’t trying to pick on anyone. I was pointing out that there is considerable room for confusion on what is a positive lapse rate and what is a negative lapse rate.
I know that Google searches are not definitive, but the first five hits for “lapse rate” say …
All of these are in the camp that “cooling as you go up is a positive lapse rate”. (This is akin to saying that “g” is +9.8 m/s^2 or “e” (which might seem to stand for the charge of an “e”lectron) is +1.6e-19 C.)
Also, you say that “They [the US Standard Atmosphere] list the lower troposphere lapse rate as -6.5 K/km”, but it never seems to actually define the “lapse rate”. They have equations and letters, but nowhere do I see anything in black and white like “the lapse rate is -6.5 K/km”. Of course, the whole pdf is sideways, so it is tough to read! If you can find such a quote, I would be happy to reconsider.
“I know that Google searches are not definitive, but the first five hits for “lapse rate” say … ”
And that is the sorry state we find ourselves in, most popular sites on the web copying off of other sites and even the units of what they are copying says they are all wrong, even just logically. But then, I bet they don’t hawk units, like in dimensional analysis, that ties together all of the other equations that use such parameters and all of those other equations units must always by consistent and correct even in signs. You just hit on one of my pet peeves.
And Tim, the definition of “lapse rate” is written right in front of you in the units that it IS. Temperature change with a change in distance. What is in question is whether up is positive distance or is it downward that is positive.
wayne,
Thank you for posting the link to http://www.astrohandbook.com/ch09/standard_atmos_1976.pdf.
It is interesting to note that standard atmosphere is an idealization selected to minimally deviate from a broad spectrum of data. (my words, not theirs) using formulae based on known physical properties and relationships to generate the values, that there were no changes to any of the 1962 standard atmosphere values up to 51 km, and that “…’…especially for heights below 20km’…” they do not represent averages of data available.
Tim Folkerts,
Read the foreword and the first paragraph of Part 1. “…”The air is assumed to obey the perfect gas law and hydrostatic equation which, taken together, relate temperature, pressure and density with geopotential.”…”
If viewing from Chrome, try right click on a page and pick rotate clockwise.
wayne,
Thank you for posting the link to http://www.astrohandbook.com/ch09/standard_atmos_1976.pdf.
It is interesting to note that standard atmosphere is an idealization selected to minimally deviate from a broad spectrum of data. (my words, not theirs) using formulae based on known physical properties and relationships to generate the values, that there were no changes to any of the 1962 standard atmosphere values up to 51 km, and that “…’…especially for heights below 20km’…” they do not represent averages of data available.
Tim Folkerts,
Read the foreword and the first paragraph of Part 1. “…”The air is assumed to obey the perfect gas law and hydrostatic equation which, taken together, relate temperature, pressure and density with geopotential.”…”
If viewing from Chrome, try right click on a page and pick rotate clockwise.
Wayne says :What is in question is whether up is positive distance or is it downward that is positive.”
No, that is not quite the question, I think everyone agrees that moving upward is positive for altitude. The question is how to write the equations and how to define “the lapse rate”. For example, we could say the lapse rate is “L” and then write either
T = T_0 + L(H – H_0) [where L is around -6.5 K/km]
T = T_0 – L(H – H_0) [where L is around +6.5 K/km]
It appears that most sources go with the second choice. This includes the American Meteorological Association’s official glossary, which says that “lapse rate” is “The decrease of an atmospheric variable with height, the variable being temperature, unless otherwise specified.”
tchannon says: September 24, 2014 at 4:15 am
“I’ve never met most earthlings. Stops to ponder, moves on falls flat on face. Dang gum on broadwalk.”
OK, sorry, Most earthlings I have had the misfortune to observe, walking, chewing.
Mysterious verb precedents intentional.
Kristian says: September 23, 2014 at 8:12 pm
Will Janoschka says, September 23, 2014 at 11:42 am:
(“Just who and what has ever measured total EMR exitance from the Earth system?
How could that possibly done without observing the whole disk of the Earth system?”)
“You have a satellite or two with IR-detecting instruments (like CERES on the Terra & Aqua satellites). They circle the Earth, measuring IR flux from Earth as they move. They make thousands of different measurements, from the poles to the equator, from the eastern to the western hemisphere. They do this for several years. Endless rounds. You end up with a mean value. This mean value closely matches the evened out incoming flux from the Sun. No wonder. They should and must balance.”
Aqua was the only satellite that measured radiance other than from nadir and all of them have a fixed FOV (steradiance). None can possibly measure the total radiand intensity from the Earth. Only a WFOV radiometer no closer than the Moon could possibly measure such! You have been fed more BS from NASA.
(“Just who and what has ever measured total Solar EMR flux absorbed by the Earth system?
Is EMR flux the only way the Earth system receives energy?”)
“Several satellites detect TSI from the Sun. To spread this flux evenly across the globe and the diurnal cycle (or over a year, for that matter), you divide it by 4.”
That is irradiance, never flux, That irradiance must be decreased by any opposing radiance from the Earth. Since most Solar irradiance is less than 2.5 microns there is little opposing radiance. That incident flux is never distributed evenly across the earths surface Solar flux at the terminator must be zero W/m2.
“Then you have to figure out Earth’s average global albedo. This you also do by satellite sensors measuring reflected radiation (SW mostly). You do it by taking thousands upon thousands of individual measurements, and then finally, after a few years, you have a mean value.”
Albedo is defined as visable backscatter from the disk of a body illuminated by its primary. It does not include any backscatter from less than 0.4 microns or more than 0.7 microns. It also does not include any of the considerable forward scattering from the atmosphere at any wavelength.
Your TSI (1-0.3)/4 is part of the BS promoted by ClimAstrologists! When will you ever learn.
“For Earth in space this is about 0.3. So you time the TSI diveded by 4 with 0.7. And what do you get? 238-240 W/m^2.”
1340 W/m^2 (TSI) x 0.5 (acceptance of atmosphere and surface) is confined to surface normals +/- 45 degrees another 0.5 of the cross-sectional area of the earth. Please show any “measurement”, not fake calculation of nonsense, that has ever been made?
“Exactly the same as the average outgoing IR flux from Earth to space. Lo and behold, they match! Balance. Did anyone expect otherwise? Nature’s fine-tuned machinery!”
Your favorite ClimAstrologists claim is 0.6 W/m^2 more in than out, CAGW, Yet you use only the ClimAstrology claims to show their claims are incorrect!
“I find this whole thing not a very controversial topic. It’s fairly straightforward. But you apparently do. I guess this is also all a part of the grand nuevo science/climastrology scam, isn’t it, Will?)
Yes it is. The controversy is why you keep eating the garbage they serve up, when you know it is garbage. Why not try to think if” why” it is continuing garbage?
(“OK, Why is it not some other temperature?”)
“What kind of question is that? Because it isn’t. That’s the temperature it needs to be at. To be able to balance incoming with outgoing. Apparently. With our current input from the Sun (included our current global albedo). And with the current mass (weight, heat capacity) of our atmosphere.”
And the variable WV in the atmosphere plays no part I suppose! What controls the current amount of atmospheric WV? Why does the Specific heat of the atmosphere have anything to do with atmospheric or surface temperature, with latent heat and radiative exitance from the atmosphere? To be continued
Kristian says: September 23, 2014 at 8:12 pm
Will Janoschka says, September 23, 2014 at 11:42 am:
Continued
(“What part of the atmosphere does this insulating? How is convective/evaporative heat transfer limited?”)
(“Again a weird question to ask. What part? All of it. The atmosphere AS A WHOLE, as a thing, does the insulation. No particular PART of it. The fact that it has a mass.”)
The WV in the atmosphere and the emissivity of that atmospheric WV and clouds provide
a more efficient way of radiating energy to space than the surface can do!
“Convective/evaporative heat loss for the surface is limited at a certain temperature level because 1) the atmosphere, having a mass, has a ‘heat capacity’, hence it is able to warm; it primarily warms convectively from the surface; it therefore sets up a certain temperature gradient away from the solar-heated surface; as you know, in convective transfer, the steepness of temperature gradients matter; and 2) the atmosphere, having a mass, also has a ‘weight’ and a density; this leads to two effects – a) the air gets ‘heavy’, which affects the buoyant acceleration (upward momentum) of air at a certain temperature, and b) the atmosphere itself presses down on the surface with a certain total weight (downward force), expressed by surface air pressure, this affects evaporation rates from especially the ocean at a certain surface temperature, which again affects buoyant uplift of air.”
Any increase in surface pressure is more than compensated by the increase in WV partial pressure do to that same increase in temperature.
“The atmosphere in principle insulates the solar-heated surface of the Earth in the same manner as everyday insulation does (like clothes, blankets and walls/ceilings), only not with a rigid blockage of air movement, rather with a restriction of free (ideal) movement. It’s all so plain and simple.”
There is no restriction especially with the great lateral winds providing increased evaporation and a fine motor for your seacraft.
(“Only if you think you know “how” this planet works!
All of your claims are those of ClimAstrologists who think they know!”)
“Yeah, that’s rich. I’m such a Climastrology fanboy. I swallow everything they serve me. You just keep on believing that, Will …”
Your own written words demonstrate that you keep on swallowing! Not one word, phrase, sentence, paragraph, chapter, or article is in any way correct from ClimAstrologists, even the punctuation is suspect.
Tim Folkerts says: September 23, 2014 at 8:33 pm
(Will asks: “Just who and what has ever measured total EMR exitance from the Earth system?”)
“See the graphs Tallbloke posted. Several satellites measure earth’s IR.”
They only measure “radiance” from a paticular direction with a very limited FOV.
“(How could that possibly done without observing the whole disk of the Earth system?”)
“Satellites *do* measure the whole disk over the course of several orbits. Combining the results at various times and places gives the total.”
There can be no correct combining of those results. At the distance of the Moon the radiant intensity of the Earth can be and has been measured!
(“Just who and what has ever measured total Solar EMR flux absorbed by the Earth system?”)
“Same answer. Satellites routinely measure insolation and albedo. (And there are various other ways to measure albedo as well). From these it is trivial to calculate the total power absorbed”
See my response to Kristian on your rediculous claim of calculation, not measurement.
(“Is EMR flux the only way the Earth system receives energy?”)
“Yes, pretty much.
* The sun averages ~ 340 W/m^2 of incoming power (with ~ 100 W/m^2 of that reflected away).
* The full moon is on the order of 0.001 W/m^2
* Geothermal is estimated to be ~ 0.1 W/m^2 up from the interior.
* I suppose you could consider solar wind & infalling meteors, but that would be even smaller.
* Some moons of Jupiter have significant tidal heating, but that would not apply to the earth.”
That is what your ClimAstrologists have assumed!
Has the earth significant Lunar tidal energy increase? Why is the Moon so cold?
The Solar radiative bulge provides how much energy? Why?
Please calculate energy transfer for:
1) Charged and neutral particle flux.
2) Additional EUV, and gamma from CME’s.
3) Changes in rotational inertia of the earth.
4) Electromotive forces.
5) Magnetomotive forces.
Why does an induction motor in a dark basement increase in temperature when doing work?
Why defend the indefensible? Nobody knows!
Tim Folkerts says: September 25, 2014 at 2:56 am
“It appears that most sources go with the second choice. This includes the American Meteorological Association’s official glossary, which says that “lapse rate” is “The decrease of an atmospheric variable with height, the variable being temperature, unless otherwise specified.”
Have been using these numbers my whole working live as you state it.
Pressure, density, temperature etc decrease with increasing height.
Calling these lapse rates negative would be like a “negative temperature increase” with height.
“Calling these lapse rates negative would be like a “negative temperature increase” with height.”
Yep, it is kind of like that! 🙂
I didn’t create this convention; I’m just relaying along how every in the field seems to use the word “lapse rate”. I have no particular attachment to one or the other, but it is important to know that this is one very common sign convention.
And think about what “lapse” itself means: “1b : a temporary deviation or fall especially from a higher to a lower state 2: a becoming less : decline ” [http://www.merriam-webster.com/dictionary/lapse]. The “lapse rate” is the rate at which some parameter FALLS or BECOMES LESS from a high state to a low state, not the rate at which it RISES.
Ben Wouters says:
September 24, 2014 at 2:1
I did hope it would be clear my question was kind of rhetorical.
I do not see how our measly atmosphere can INCREASE the temperature of especially the (deep) oceans
your question was:
If the pressure of the atmosphere can increase the temperature of the surface some 80 or 90K above that of the moon, why isn’t the temperature increasing with increasing depth of the oceans?
I think it is a good question because it what everyone thinks. Oceans are denser and heavier than air. They cause a big pressure gradient pressure but there is no big temp.gradient in the ocean. The atmosphere has a smaller pressure gradient. It cannot cause the LR.
I do not say pressure causes LR
I say acceleration causes LR
acceleration x distance = velocity
0.003g x 10km = 300m/s2
According to Kinetic Theory,T depends only on velocity
In water 100% of radiation is absorbed in 150m. Change of g in 150m is very small.So no LR in oceans
I see my answer to your question, rhetorical or not, as support for my theory.
Another interesting question is what happens to LR underground, in deep mines?
What are the facts and what is your explanation?
What do you mean by “static atmosphere?
Roger says: “acceleration x distance = velocity
0.003g x 10km = 300m/s2”
Acceleration x time = velocity (when starting at rest)
So the whole concept is wrong to start with. The units should be a big clue. First of all, your units of m/s^2 are not correct units for velocity (that would be an acceleration). Furthermore, the answer would actually be 300 m^2/s^2, so the units should be the units for velocity squared.
And what does 0.003g represent, anyway? What do you think is accelerating at 0.0294 m/s^2?
I took a bit of time out because I was not getting the true simplicity of reality across.
I see that a number of subsequent commenters are getting very close.
At its simplest the issue is one of radiation balanced with conduction/convection so as to maintain thermal equilibrium over time despite variations about the mean.
Convection will always change in order to balance radiation and conduction so that the surface temperature remains determined by mass, gravity and insolation alone.
The phase changes of water simply help the process along.
The radiative capability of atmospheric molecules allows energy to escape to space directly from within the atmosphere and in doing so reduces the amount of energy returned to the surface in adiabatic descent.
More energy goes out from within the atmosphere and less goes out from the surface if one increases GHGs and the two changes offset one another exactly so as to keep the surface temperature the same and the system stable.
That’s all there is to it.
Tim Folkerts says:
September 25, 2014 at 4:58 pm
Roger says: “acceleration x distance = velocity
0.003g x 10km = 300m/s2″
Acceleration x time = velocity (when starting at rest)
So the whole concept is wrong to start with. The units should be a big clue. First of all, your units of m/s^2 are not correct units for velocity (that would be an acceleration). Furthermore, the answer would actually be 300 m^2/s^2, so the units should be the units for velocity squared.
And what does 0.003g represent, anyway? What do you think is accelerating at 0.0294 m/s^2?
You are right.
v = accel. x time, not
v = accel x distance
I should use
v^2 = u^2 +2gs
= 0 + 2 x 0.003 x 10 x 10 000
= 600
v = 25m/s
0.003g = 0.003 x 10m/s2
= 0.03m/s2
This is the difference in gravity between the surface and at 10 000m.
My calculation is for a thought experiment. To demonstrate that anything moving from 10 000m to the surface will gain 25m/s.
This is of the same order as the difference in vel. of molecules at 220K and 290K.
ave v at 290K = 500m/s
ave v at 220K = 450m/s
difference 50m/s
Gravity alone can cause the GHE.
“My calculation is for a thought experiment. To demonstrate that anything moving from 10 000m to the surface will gain 25m/s.”
Motion is based on the actual acceleration, not on some change in acceleration. Any object freely falling from 10,000 m to the surface will be going
v = (2gh)^0.5 = 440 m/s = 1600 km/hr (NOT ~ 25 m/s)
There will be a small correction due to the fact the g is slightly smaller at 10,000 m, but this will be minor. If g changes by 0.3%, then final speed will change by a similar (but smaller amount). This correction is almost certainly less than a few m/s.
The corrections due to any air resistance will be dramatically more important.
Tim Folkerts says: September 25, 2014 at 7:50 pm
(“My calculation is for a thought experiment. To demonstrate that anything moving from 10 000m to the surface will gain 25m/s.”)
“Motion is based on the actual acceleration, not on some change in acceleration. Any object freely falling from 10,000 m to the surface will be going
v = (2gh)^0.5 = 440 m/s = 1600 km/hr (NOT ~ 25 m/s)
There will be a small correction due to the fact the g is slightly smaller at 10,000 m, but this will be minor. If g changes by 0.3%, then final speed will change by a similar (but smaller amount). This correction is almost certainly less than a few m/s.
The corrections due to any air resistance will be dramatically more important.”
Rainfall is limited to less than 10 m/s. Large hail can hit 30 m/s.
wildeco2014 says: September 25, 2014 at 6:58 pm
“I took a bit of time out because I was not getting the true simplicity of reality across.
I see that a number of subsequent commenters are getting very close.
At its simplest the issue is one of radiation balanced with conduction/convection so as to maintain thermal equilibrium over time despite variations about the mean.
Convection will always change in order to balance radiation and conduction so that the surface temperature remains determined by mass, gravity and insolation alone.”
Why do you claim that?
“The phase changes of water simply help the process along.”
WV and condensation alone control radiative exitance from the atmosphere.
“The radiative capability of atmospheric molecules allows energy to escape to space directly from within the atmosphere and in doing so reduces the amount of energy returned to the surface in adiabatic descent.”
Please show that any energy heat or internal is returned to the surface? The virial theorem does not apply directly in the troposphere.
“More energy goes out from within the atmosphere and less goes out from the surface if one increases GHGs and the two changes offset one another exactly so as to keep the surface temperature the same and the system stable. That’s all there is to it.”
If one increases WV, surface radiation is completely replaced by atmospheric radiative exitance.
@Tim Folkerts: 24, 2014 at 4:24 pm
“All the sources that I checked concur that a POSITIVE lapse rate mean DECREASING temperature as you go up. So while most physicists would call this a “negative gradient”, meteorologist use the opposite sign convention. An inversion has a negative lapse rate.
Using the “standard” meteorological definition, the theoretical lapse rate in the atmosphere is POSITIVE, not negative as you have said a few times.”
You are right that the L = -g/Cp means that temperature rises as you go up. I have made some sloppy mistakes this week because I am working away from home and don’t have time to double check everything. When I am not working my mistakes are due to an excess of Glenfiddich 12 year old malt whisky or the lateness of the hour.
Years ago I used to include deliberate mistakes in my presentations to find out whether my students were paying attention. I don’t do that any more as I make so many unintentional mistakes. In my defense I am usually prepared to admit error unlike Michael Mann who has yet to retract his papers with the inverted Tiljander sediments.
You might enjoy this exchange with a pompous ass called David Appell who failed to spot one of my deliberate “schoolboy howlers”:
Appell was right about one thing. I make careless mistakes so “sloppy” is probably a fair comment.
gallopingcamel says: September 26, 2014 at 2:21 am
“You might enjoy this exchange with a pompous ass called David Appell who failed to spot one of my deliberate “schoolboy howlers”:
Appell was right about one thing. I make careless mistakes so “sloppy” is probably a fair comment.”
Peter that was a hoot! I was amused when David claimed the ClimAstrologists use the HiTran database. They only misuse it. One could calculate lots from HiTran in 1974. One thing you can not use it for directly is for the attenuation of flux, as it was created to predict the attenuation spatial modulation from a thermal “scene”. From 64 to 74, no one cared about flux, only what you can see!
TimF:
Ok, I give. I’ll go back and change the signs in all of my hydrostatic equations in programs to assume a reversed frame of reference of negative z pointing upward in the values and make my lapse rate to be positive so temperatures are decreasing upward from the surface. Wait, I don’t really have to do that if I’ll just also change my gravity acceleration value to also be a positive. (just kidding, I like altitudes to stay positive so I’ll leave it as it is)
Tim, I must admit you are very good and persistent arguer, which I am not so. Maybe instead you could take the lead and wander over to Wikipedia and have them correct their “Lapse Rate” page for according to your preference they are all messed up too and say:
I guess I have just become a bit of a purist over the years for I would have been fired on the spot for such ambiguities.
Like most others I so often just use the term 6.5 K/km assuming everyone knows it’s literally a negative if +z is upward so I will start being a bit clearer when speaking of such values. Also, you could just move that ‘minus’ out from the value and put it between the terms as you did above but that is where I was trained to never, ever to do such antics for you then have assumed someone else ‘knows’ you have rigged the equation to ignore the frame of reference in the values.
If everyone here is a meteorologist I’ll follow suit for I already know how to interpret such use of signs in their equations, it’s quite obvious when they occurs.
gallopingcamel:
Good you caught that minus in L = -g/cp. I did noticed you used it on another site a while back and that is not consistent and correct in either frame of reference, +z or -z being upward.
“Ok, I give. I’ll go back and change the signs in all of my hydrostatic equations in programs to assume a reversed frame of reference of negative z pointing upward …”
No, that is not the solution! Positive z still points upward.
“Maybe instead you could take the lead and wander over to Wikipedia and have them correct their “Lapse Rate” page for according to your preference they are all messed up too and say …
\displaystyle \gamma = -\frac{dT}{dz}
This is correct (using the standard definitions of “lapse rate”) so there is no need to change it. dT/dz is the slope of T as your change z (where z is indeed positive as you go upward). This slope happens to be negative (under normal conditions). When dT/dz is itself negative, then -dTdz is positive, and gamma is positive.
Roger Clague says: September 25, 2014 at 1:12 pm
“I say acceleration causes LR”
The ELR (atmospheric temperature profile) is NOT caused by acceleration.
It is a measure of how fast the atmosphere lets the energy from the solar heated surface escape to space.
“Another interesting question is what happens to LR underground, in deep mines?”
Going down in the crust the temperature increases ~25K/km due to geothermal heat.
see eg http://en.wikipedia.org/wiki/TauTona_Mine
and
http://en.wikipedia.org/wiki/Geothermal_gradient
“What do you mean by “static atmosphere?”
In discussing convection it is assumed that the surrounding air is static.
At least not moving up or down. You can still do convective calculations in a wind environment, as long as the wind is horizontal.