Apologies for the language mash-up. If I can find a better way to scrape the translation without getting the original Russian too, I’ll update it. This looks interesting, and relevant to recent discussions on the Jelbring and Nikolov and Zeller theories. Good luck. H/T’s Lucy Skywalker and Yankov
Temporary, added English translation PDF via Lucy’s doc/odt files sorokhtin.pdf[ –Tim
[UPDATE} better formatting now in place – Thanks a million Tim!
Marathon-2005
Academician (RANS) OG Sorokhtin,
Institute of Oceanology. Shirshov Sciences
The adiabatic theory of greenhouse effect
The idea of ??heating the earth’s atmosphere by greenhouse gases was first expressed in the late XIX century, the famous Swedish scientist Arrhenius S. [1] and since the obvious is taken for granted, with little or no verification [2-5]. This view is now completely dominates the conclusions of the Intergovernmental Panel on Climate Change (IPCC), Greenpeace, the UN Environment Programme (UNEP), World Meteorological Organization (WMO), as well as the withdrawal of Russian environmental and scientific organizations. The same view was fully supported by the decisions of international environmental conventions, in Rio de Janeiro (Brazil) in 1992 and in Kyoto (Japan) in 1997 is projected proponents of these ideas, by 2100, warming could reach 2.5 -5 ° C and cause sea level rise of 0.6 m -1, which already may be a problem for densely populated areas of the continental coasts, as well as for gas and oil production in lowland areas and most of the coasts of northern Russia. Projected, and other harmful consequences for the nature of global warming (the expansion of deserts, the disappearance of permafrost, soil erosion, etc.).
Fears of similar catastrophic events, the pressure of environmental organizations, and often simply speculation on this subject makes governments of developed countries to allocate huge resources to fight the effects of global warming, allegedly linked to anthropogenic emissions to the atmosphere of “greenhouse gases”. And how justified are these costs? Not if we’re fighting “quixotic”?
On closer acquaintance with this problem it turned out that the theory of greenhouse effect as such until the 90s. the last century did not exist, and all calculations of the effect of CO 2 and other greenhouse gases in the Earth’s climate was carried out on different intuitive models with the introduction of numerous and not always stable parameters [4]. In this case the uncertainties in the estimates of various parameters of the model adopted (and they number at least 30) actually make the solution of the problem incorrectly. We decided to use a synergistic approach [6, 7] and an analysis of the most common positions, representing the Earth’s atmosphere as an open dissipative (scattering power) system described by nonlinear equations of mathematical physics.
The main characteristics of the atmosphere
The mass of the modern atmosphere is about 5.15. 1021 g, average air pressure at sea level, p 0 is the same physical environment, or 101.32 kPa, the density  With high air pressure rapidly decreases exponentially (Fig. 1):
With high air pressure rapidly decreases exponentially (Fig. 1):
 (1)
(1)
where g = 9,81 m / s 2 – acceleration due to gravity;  – The average molar mass of atmospheric gases (equal to 28.97 g / mol at p = 0), R = 1,987 cal / (K. Mole) = 8.314. July 10 erg / (K. Mole) – gas constant, T – absolute temperature; h – the height above sea level. Accordingly, decreases with height and density of air.
– The average molar mass of atmospheric gases (equal to 28.97 g / mol at p = 0), R = 1,987 cal / (K. Mole) = 8.314. July 10 erg / (K. Mole) – gas constant, T – absolute temperature; h – the height above sea level. Accordingly, decreases with height and density of air.

Dry air contains 75.51% (by mass) of nitrogen, 23.15% oxygen, 1.28% argon, 0.046% carbon dioxide, neon, 0.00125% and about 0.0007% other gases. The important active component is water vapor (and water droplets in clouds): their average mass reaches 0.13. 10 20 g, equivalent to a layer of condensed water is 25 mm, an average of 2.5 g / cm 2. Considering the average annual evaporation and precipitation, which is approximately 780 mm of water column, it is easy to determine that the water vapor in the atmosphere is updated approximately 30 times a year, or every 12 days. In the upper atmosphere under the influence of solar ultraviolet radiation arises ozone (O 3). Despite the small amount (about 3.1. 10 15 g, while the oxygen 1.192. 10 21 g), this gas saving life on Earth from harmful solar radiation hard.
We can distinguish three characteristic layer of atmosphere (Fig. 2) [8]. The bottom and most dense layer – the troposphere – which extends to a height of about 8-10 km in high latitudes and up to 16-18 km in the equatorial belt (on average – up to 12 km), contains about 80% of the mass of the atmosphere and is characterized by a nearly linear distribution the temperature. The middle layer is essentially a rarefied atmosphere include the stratosphere and mesosphere and is characterized by a sharp maximum temperature reaching 270 K at an altitude of 50 km. Even higher is the thermosphere, in which the temperature of the ionized gas increases with altitude up to 1000 K or more, and at altitudes above 1000 km, the thermosphere is gradually transformed into the exosphere and beyond – into space. Between the troposphere and stratosphere, mesosphere and thermosphere are transition layers – respectively the tropopause (the temperature of about 190-220 K) and the mesopause (about 180-190 K).
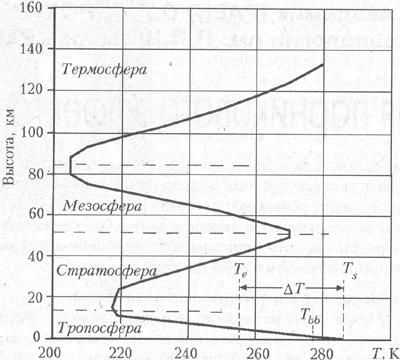
In Fig. 2. The temperature distribution in the Earth’s atmosphere: T e – radiation (effective) temperature of the Earth; T s – the average temperature on Earth, reduced to sea level;  T – the value of the greenhouse effect; T bb – the temperature of a blackbody, orbiting the Earth
T – the value of the greenhouse effect; T bb – the temperature of a blackbody, orbiting the Earth
In the troposphere, the temperature increases with height almost linearly, whereas in the upper atmosphere has a sharp maximum at an altitude of 50 km and rise to altitudes above 90 km. Maximum is associated with the absorption of ultraviolet solar radiation by ozone, increased due to the ionization of the rarefied air rigid solar radiation.
Thus, in the stratosphere and mesosphere temperature is mainly determined by radiative heat transfer mechanism, whereas in the troposphere – the other processes, chief among which is the convective heat loss from the lower, dense layer in the stratosphere, where it lost to space by radiation already.
The adiabatic theory of greenhouse effect
By definition, the greenhouse effect  T is the difference between the mean surface temperature T s and its radiation (effective) temperature T e, under which the planet is visible from space:
T is the difference between the mean surface temperature T s and its radiation (effective) temperature T e, under which the planet is visible from space:
 T = T s – T e. (2)
T = T s – T e. (2)
The average temperature across the Earth as a whole is approximately equal to 288 K (+15 ° C), and its effective temperature is defined as follows:
 (3)
(3)
where  = 5.67. 10 -5 erg / (cm 2. With. K 4) – Stefan-Boltzmann constant, S – the solar constant at the distance of the planet from the Sun, A – albedo, or reflectivity, of the planet, largely controlled its cloud the cover. For the Earth, S = 1,367. June 10 erg / (cm 2. With), A
= 5.67. 10 -5 erg / (cm 2. With. K 4) – Stefan-Boltzmann constant, S – the solar constant at the distance of the planet from the Sun, A – albedo, or reflectivity, of the planet, largely controlled its cloud the cover. For the Earth, S = 1,367. June 10 erg / (cm 2. With), A  0,3, T e = 255 K (-18 ° C), and hence the greenhouse effect on Earth right now is +33 ° C.
0,3, T e = 255 K (-18 ° C), and hence the greenhouse effect on Earth right now is +33 ° C.
In terms of this definition the greenhouse effect is very real category, although the term greenhouse effect is unsuccessful and physically just wrong. It is believed that the atmosphere contains so-called “greenhouse gases”, weakly absorbs solar shortwave radiation, which for the most part reaches the earth’s surface but delays the long-wave reradiated by this surface (thermal) radiation, thus greatly reducing heat transfer from Earth to space. This is taken as the main cause of increasing temperature. The higher the concentration in the air referred to “greenhouse gases”, those considered to be more warming of the atmosphere. His name was on the effect of events in greenhouses covered with glass roofs (greenhouse effect), because the glass is too easily lets solar radiation in the visible spectrum, but delays the infrared. However, the main effect of greenhouses and polytunnels in the other – to prevent convective mixing filling their air with outside air, as soon as opening windows and greenhouses restored communication with the outside space, once lost, and the “greenhouse effect”.
Because the Earth has a relatively dense atmosphere, the bottom and most dense layer of her – the troposphere – the heat transfer is mainly due to convective motions of air masses, and not only on the mechanism of radiation – the radiation path, as it is imagined by proponents of the classical approach. Indeed, in the dense troposphere (pressure greater than 0.2 atm), the warm air mass expands and rises, and cold, on the contrary, shrink and fall. Radiative heat transfer is dominant only in the rarefied stratosphere, mesosphere and thermosphere.
Hence the main conclusion: the average temperature distribution in the troposphere should be close to adiabatic, ie established with the expansion and cooling of air in its ascent, and, conversely, the compression and heating during lowering. (Specific distribution of temperature at specific points in time need not be adiabatic. We have in mind only the average distribution of time intervals of the order of months).
In an adiabatic process is the absolute temperature by the equation [9] (assuming an ideal gas atmosphere):
 (4)
(4)
where C – constant, p – pressure of the gas mixture  – Ratio of specific heats,
– Ratio of specific heats,  c p and c V – specific (per unit mass) heat capacity of gas, respectively, at constant pressure and constant volume. For all of triatomic gases (including CO 2 and H 2 O)
c p and c V – specific (per unit mass) heat capacity of gas, respectively, at constant pressure and constant volume. For all of triatomic gases (including CO 2 and H 2 O)  = 1.3,
= 1.3,  = 0.2308, and for diatomic (N 2 and O 2)
= 0.2308, and for diatomic (N 2 and O 2)  = 1.4,
= 1.4,  = 0.2857. The condensation of water vapor in the troposphere and the absorption of moisture, “greenhouse” gases, the infrared radiation is heat and rising temperatures. This leads to a change in
= 0.2857. The condensation of water vapor in the troposphere and the absorption of moisture, “greenhouse” gases, the infrared radiation is heat and rising temperatures. This leads to a change in  . For example, the average value of this parameter for a wet, absorbing infrared radiation of the earth’s troposphere is equal to 0.1905 [10], whereas for the dry air is 0.2846.
. For example, the average value of this parameter for a wet, absorbing infrared radiation of the earth’s troposphere is equal to 0.1905 [10], whereas for the dry air is 0.2846.
It is important to note that the moisture in the troposphere generates clouds, which is the main factor determining the Earth’s albedo. This creates a strong negative feedback between surface temperature and radiation, which leads to stabilization of the temperature regime of the troposphere (Fig. 3). Indeed, any increase in surface temperature increases evaporation and increases the cloudiness of the Earth, and this in turn increases the planetary albedo and reflectivity of the Earth’s atmosphere. As a result of increased reflection of solar heat from the clouds into space, reducing its supply to the Earth, and the average surface temperature is reduced again to its previous level. Keep in mind that any negative feedback in the system leads to a linear response to output from the effects of the input [11]. This property is manifested regardless of the nature of the systems themselves, whether it is the planet’s atmosphere, or the electronic amplifier Watt centrifugal governor.

When the input signal is the so-called blackbody temperature, which characterizes the heat of the body remote from the Sun to the Earth-Sun distance, only by the absorption of solar radiation (T bb = 278,8 K = +5,6 ° C for the earth), then mean surface temperature T s is linearly dependent on it. Consequently, the average temperature at any level of the Earth’s troposphere
 (5)
(5)
where b – a scale factor (if measurements are carried out in the physical atmosphere, the Earth for b = 1,186 atm-1). Since the average surface temperature of Earth is 288 K, then from (4) it immediately follows that at any level in the Earth’s troposphere (at p> 0,2 bar)
 (6)
(6)
where p 0 = 1 atm – atmospheric pressure at sea level (hereafter the subscript “0” marked the modern values ??of the parameters of the atmosphere).
Equation (5) can be used in the case of other planets, if we consider the dependence of the Stefan-Boltzmann law:
 (7)
(7)
Then for any planet with a dense (with p> 0,2 bar) atmosphere we
 (8)
(8)
Dependence ![]() the composition and humidity of the atmosphere can be easily found by the known formula:
the composition and humidity of the atmosphere can be easily found by the known formula:
 (9)
(9)
 (10)
(10)
where 
![]() 29 g / mol – molar mass of air;
29 g / mol – molar mass of air;  = 76.49 kPa
= 76.49 kPa  = 23.45 kPa
= 23.45 kPa  = 0.046 kPa, p Ar = 1,297 kPa – the partial pressure of the gas [12]; p = 101,3 kPa – the total pressure of the atmosphere, with p (N 2) = 0.248 cal / (g. K), with p (O 2 ) = 0.218 cal / (g. K), c p (CO 2) = 0.197 cal / (g. K), with p (Ar) = 0,124 cal / (g. K) [13], with w and r – correction factor having the dimension of the specific heat and taking into account respectively the total heat effect of moisture condensation processes in a humid atmosphere (a w) and the absorption of thermal radiation of the Earth and the Sun (with r).
= 0.046 kPa, p Ar = 1,297 kPa – the partial pressure of the gas [12]; p = 101,3 kPa – the total pressure of the atmosphere, with p (N 2) = 0.248 cal / (g. K), with p (O 2 ) = 0.218 cal / (g. K), c p (CO 2) = 0.197 cal / (g. K), with p (Ar) = 0,124 cal / (g. K) [13], with w and r – correction factor having the dimension of the specific heat and taking into account respectively the total heat effect of moisture condensation processes in a humid atmosphere (a w) and the absorption of thermal radiation of the Earth and the Sun (with r).
If p = 0.2394 cal / (g. K) to dry the earth’s atmosphere, then  = 0.1905 for wet and absorbing infrared radiation of the atmosphere averaged with w + r = 0,1203 cal / (g. K). For planets with atmospheres of different nature under these parameters should be understood as characteristic of any thermal or chemical processes that lead to the release or absorption (with w + with r <0) of heat in the troposphere.
= 0.1905 for wet and absorbing infrared radiation of the atmosphere averaged with w + r = 0,1203 cal / (g. K). For planets with atmospheres of different nature under these parameters should be understood as characteristic of any thermal or chemical processes that lead to the release or absorption (with w + with r <0) of heat in the troposphere.
Verification of the adiabatic theory of greenhouse effect by comparing the conduct of theoretical and experimental temperature distributions in the troposphere of the Earth and Venus. First we find the values ??of the corresponding adiabats. To do this, we substitute the expression (8) the parameters of the atmosphere of the Earth:
S 0 = 1.37. 10 6 erg / (cm 2. A); (T bb) 0 = 278.8 K; (T s) 0 = 288 K; p 0 = 1 atm. Then the expression (5) we find  = 1.033, the values ??of temperature and pressure at an intermediate level (eg, at an altitude of 5 km: T = 255.5 K 5km, 5km p = 0.5333 atm) and the expression (6) we find
= 1.033, the values ??of temperature and pressure at an intermediate level (eg, at an altitude of 5 km: T = 255.5 K 5km, 5km p = 0.5333 atm) and the expression (6) we find  = 0.1905 and b = 1,186 atm -1. Calculations coincide with the actual distribution of temperature in the troposphere standard atmosphere [14] with an accuracy of ± 0.1% (Fig. 4). Recall that the standard model of the Earth’s atmosphere is essentially a homogenized across the earth temperature and pressure dependence of the height above sea level. This model of the troposphere with a gradient of 6.5 K / km is usually used to configure the aircraft altimeter and barometer calibration, designed for ground-based observations.
= 0.1905 and b = 1,186 atm -1. Calculations coincide with the actual distribution of temperature in the troposphere standard atmosphere [14] with an accuracy of ± 0.1% (Fig. 4). Recall that the standard model of the Earth’s atmosphere is essentially a homogenized across the earth temperature and pressure dependence of the height above sea level. This model of the troposphere with a gradient of 6.5 K / km is usually used to configure the aircraft altimeter and barometer calibration, designed for ground-based observations.

Much tougher test of the universality of the laws is derived calculation of temperature distribution in a dense carbon dioxide troposphere of Venus for a given pressure and composition of its atmosphere: p s = 90,9 bar; T s = 735,3 K and S = 2,62. June 10 ergs / (cm 2. c) [15, 16]. Arguing similarly, and substituting T = 496.9 K 30km, 30km p = 9.458 atm, we find:  = 0,173, b = 1,167 atm -1. The best agreement between the theoretical curve with the empirical data is obtained by taking the Venus, like Earth, b = 1,186 atm -1. Calculated and experimental data on Venus match up to an altitude of 40 km with an accuracy of about 0.5-1.0%. Above 60 km, with p <0.2 atm, and begins the tropopause, and the theory considered here will not work. It can be assumed that the value of the constant b = 1,186 atm -1 – universal for planets with different chemical composition of the troposphere.
= 0,173, b = 1,167 atm -1. The best agreement between the theoretical curve with the empirical data is obtained by taking the Venus, like Earth, b = 1,186 atm -1. Calculated and experimental data on Venus match up to an altitude of 40 km with an accuracy of about 0.5-1.0%. Above 60 km, with p <0.2 atm, and begins the tropopause, and the theory considered here will not work. It can be assumed that the value of the constant b = 1,186 atm -1 – universal for planets with different chemical composition of the troposphere.
Thus, the average temperature at any level in a dense planetary troposphere (pressures above 0.2 atm) is uniquely determined by the intensity of solar radiation, atmospheric pressure at this level and the effective specific heat of the gaseous medium, taking into account the additional heating (cooling) of the troposphere due to take place in It processes the selection (absorption) of heat.
Determination of moisture absorption and air
After checking the validity of the adiabatic theory of greenhouse effect, you can perform a number of predictive calculations. The model under consideration allows us to estimate the proportion of all parts of the heat transfer in the overall tropospheric temperature adjustment. Thus, according to the characteristic temperatures of the Earth’s troposphere (T e = 255 K, T s = 288 K) can not determine the correction terms to the specific heat is dry and does not absorb infrared radiation of the atmosphere, taking into account radiation from the r and w vlagokondensatsionnogo with heat transfer [10]. Let Q a – an effective thermal margin of the atmosphere, m a – its effective mass. Then the radiation component of heat capacity expressed in terms of radiation temperature by the simple relation
 (11)
(11)
Similarly, we can assume that the additional heating of the atmosphere from the radiation temperature of the planet to its mean surface temperature is characterized by the total heat capacity
 (11 ‘)
(11 ‘)
so we can write:
 (12)
(12)
or, using (9):
 (13)
(13)
 (14)
(14)
Substituting into (13) and (14) above the Earth’s atmosphere parameters, we find with r = 0,0412 cal / (g. K), with w = 0,0791 cal / (g. K), with r + c w = 0,1203 cal / (g. K), ie the same meaning as in the analysis of the average temperature distribution in the troposphere of the Earth. This again confirms the correctness of the theory. In Fig. 5 schematically shows the contributions to the heat flow from Earth into space: due to the direct transfer of heat from the earth’s surface for convective mass transfer troposphere – about 67%, due to absorption of infrared radiation of the Earth and the Sun – 11% due to condensation in the troposphere – another 22%.

The dominance of the convective component of heat removal from the troposphere to be explained naturally. Indeed, the energy absorbed by “greenhouse” gases, the infrared radiation excites oscillations in the gas molecules, which determine the heating of the irradiated volume of the gas. Further heat transfer can occur by diffusion and convection. However, the thermal conductivity of air only small – about 5.3. 10 -5 cal / (cm. With. K), which provides a heat transfer rate in centimeters per second, whereas due to convection, it can reach meters per second. The situation is similar on heating of air due to condensation of moisture in it.
To Venus (  = 0.173,
= 0.173,  = 43.2 g / mol, with p = 0.199 cal / (g. K), T s = 735,3 K, T e = 228 K) corresponding to the specific heat in cal / (g. K) are equal to r = 0 , 1834, with w =- 0,1166, with q = 0,0668. The increased value of r, determined by radiative heat transfer, appears to be due solely to the hot state it in the troposphere. A negative value means the CW, especially in the lower and middle troposphere is dominated by the endothermic dissociation reaction of some chemical compounds (for example, of sulfuric acid to sulfur trioxide and water). In the upper troposphere, at altitudes of 40 to 50 km and 60 km (with w> 0), is dominated by exothermic reaction of the formation of chemical compounds and condensation.
= 43.2 g / mol, with p = 0.199 cal / (g. K), T s = 735,3 K, T e = 228 K) corresponding to the specific heat in cal / (g. K) are equal to r = 0 , 1834, with w =- 0,1166, with q = 0,0668. The increased value of r, determined by radiative heat transfer, appears to be due solely to the hot state it in the troposphere. A negative value means the CW, especially in the lower and middle troposphere is dominated by the endothermic dissociation reaction of some chemical compounds (for example, of sulfuric acid to sulfur trioxide and water). In the upper troposphere, at altitudes of 40 to 50 km and 60 km (with w> 0), is dominated by exothermic reaction of the formation of chemical compounds and condensation.
Possible areas of expansion of the theory
The model described the greenhouse effect, is essentially a “one-dimensional ‘: the planet is a dimensionless point, the only dimension – height. Such a synergetic model is most accurate in determining the global characteristics of the troposphere of the planet, for example, the greenhouse effect, the average temperature distribution, the mean values ??of radiation or heat vlagokondensatsionnoy components, etc. Using Lambert’s law coverage areas and by considering the breadth of areas  , This model can be transformed into two-dimensional, and introducing into it the longitudinal component of the seasonal variations of light and the world – in three-and four-dimensional. The accuracy of determining the dependence of the greenhouse effect on the composition of the atmosphere decreases.
, This model can be transformed into two-dimensional, and introducing into it the longitudinal component of the seasonal variations of light and the world – in three-and four-dimensional. The accuracy of determining the dependence of the greenhouse effect on the composition of the atmosphere decreases.
In this case, the physical definition of temperature blackbody temperature should be replaced by the concept of a “gray body”:
 (15)
(15)
Now if we take into account the existence of convective heat transfer in the troposphere, the temperature of the Earth simulating a “gray body” can be written as:
 (16)
(16)
where  = DQ / dt – rate of heat transfer of air masses, such as cyclones (in this case, however, have to take into account the transfer of air masses, which may violate the adiabatic temperature distribution in the troposphere, although the relative energetics of these processes and small). At night, the S = 0, and in addition to heat transfer by air masses should take into account the rate of radiation heat warmed the day the earth’s surface. The temperature of the earth’s surface is in this approximation is equal to
= DQ / dt – rate of heat transfer of air masses, such as cyclones (in this case, however, have to take into account the transfer of air masses, which may violate the adiabatic temperature distribution in the troposphere, although the relative energetics of these processes and small). At night, the S = 0, and in addition to heat transfer by air masses should take into account the rate of radiation heat warmed the day the earth’s surface. The temperature of the earth’s surface is in this approximation is equal to
 (17)
(17)
which allows to determine the latitudinal zonation of values ??of surface temperatures. If, on the contrary, given the latitudinal distribution of empirically measured average temperatures, it is possible to determine the rate and the average specific heat addition of air masses at this latitude. From Fig. 6 shows good agreement between theoretical and empirical [5] of the average air temperature at the surface of the latitude.

The intensity of solar radiation in terms of the entire Earth’s surface is approximately equal to 1.11. October 24 erg / s, and taking into account the albedo (  0.3) until the surface reaches about 7.76. October 23 erg / sec. The average total power of tropospheric synoptic processes on the Earth about 3.79. October 23 erg / s, which corresponds to almost 50% of the power of solar radiation incident on the surface of the Earth. Obviously, at this energy the background of energy production by all mankind is negligible 13. 10 12 watts = 1.3. 10 20 erg / sec. That is why the anthropogenic influence on global energy climate of the Earth can be safely ignored. In this same connection it is interesting to note that the heat reserve of the earth’s atmosphere, according to our estimates, about 1.3. October 31 erg, the ocean heat reserve of about 1.6. 10 34 erg (amount of heat energy in the “solid” Earth about 1.6. 10 38 erg), whereas heat production by all mankind – only 4.1. 10 27 erg / year. When the snow cover from albedo A s> A surface temperature will be:
0.3) until the surface reaches about 7.76. October 23 erg / sec. The average total power of tropospheric synoptic processes on the Earth about 3.79. October 23 erg / s, which corresponds to almost 50% of the power of solar radiation incident on the surface of the Earth. Obviously, at this energy the background of energy production by all mankind is negligible 13. 10 12 watts = 1.3. 10 20 erg / sec. That is why the anthropogenic influence on global energy climate of the Earth can be safely ignored. In this same connection it is interesting to note that the heat reserve of the earth’s atmosphere, according to our estimates, about 1.3. October 31 erg, the ocean heat reserve of about 1.6. 10 34 erg (amount of heat energy in the “solid” Earth about 1.6. 10 38 erg), whereas heat production by all mankind – only 4.1. 10 27 erg / year. When the snow cover from albedo A s> A surface temperature will be:
 (18)
(18)
To generalize the model for three-dimensional version must still add the longitudinal angle, as well as identify areas of the oceans and continents. Finally, the four-dimensional model will require consideration of the angle of inclination of the Earth rotation axis to the ecliptic plane, including seasonal and diurnal variations in the illumination of the Earth, etc.
As follows from (18), the adiabatic theory of greenhouse effect explains such phenomena as the cooling of the surface layers of the air on clear nights under the anticyclones, when S = 0, and the addition of heat is small. Indeed, in the areas of anticyclones usually noticeably slow the convective mass transfer of air, therefore, reduced and convective heat flow, although the radiation of heat saved the day warmed the earth’s surface. This decrease in the factor dQ / dt in equation (18) during the night (S  0), significantly reduced surface temperature. In the same winter high latitudes where the Earth’s surface is covered with a layer of snow with high albedo and heating by solar radiation is negligible, this phenomenon leads to supercooling air and offensive “a killing frost.” When standing stable anticyclones (dQ / dt
0), significantly reduced surface temperature. In the same winter high latitudes where the Earth’s surface is covered with a layer of snow with high albedo and heating by solar radiation is negligible, this phenomenon leads to supercooling air and offensive “a killing frost.” When standing stable anticyclones (dQ / dt  0) in the snowy regions of the troposphere, there is a general hypothermia, and the tropopause descends almost to the earth’s surface. Obvious examples are the conditions arising in the central regions of Antarctica, which I have repeatedly witnessed, as well as in winter in Yakutia and Verkhoyansk. But as soon as the anticyclonic regime in the troposphere is replaced by a cyclonic, immediately restored the convective mixing of air masses, there is warming, and an average of approximately newly restored considered here the adiabatic temperature distribution.
0) in the snowy regions of the troposphere, there is a general hypothermia, and the tropopause descends almost to the earth’s surface. Obvious examples are the conditions arising in the central regions of Antarctica, which I have repeatedly witnessed, as well as in winter in Yakutia and Verkhoyansk. But as soon as the anticyclonic regime in the troposphere is replaced by a cyclonic, immediately restored the convective mixing of air masses, there is warming, and an average of approximately newly restored considered here the adiabatic temperature distribution.
Thus, this model may allow to obtain and local climatic features of the planet, for which it should enter the albedo of the earth’s surface, addition of heat and humidity of the troposphere by cyclones. In fact, in areas with high reflectivity of snow cover, deprived of heat addition of cyclones, surface temperature is reduced almost to the temperature of the tropopause is determined by the radiation balance of the atmosphere already at this latitude. In the summer time in the anticyclonic areas with dry air, on the contrary, there is overheating of surface layers of the troposphere by about 4-5 ° C or higher (with all the symptoms of drought), which is often the case, for example, in the Trans-Volga steppes.
Some forecasts
According to expression (7) can be constructed to determine the temperature distribution and its gradient is completely dry and completely transparent troposphere of the Earth. In this case, with w + r = 0 and expressions (9) and (10) we find with p = 0.2394 cux cal / (g. K) ![]() 1.0023. July 10 erg / (g. K),
1.0023. July 10 erg / (g. K), ![]() = 0.286. Then dry the troposphere the temperature gradient is
= 0.286. Then dry the troposphere the temperature gradient is
cux grad T = g / c p ![]() 9.8 K / km. (19)
9.8 K / km. (19)
Wet and warm the Earth’s troposphere, absorption, where w + r with ![]() 0.1203 cal / (g. K) = 0.504. July 10 erg / (g. K), the temperature gradient is from the expressions (13) and (14):
0.1203 cal / (g. K) = 0.504. July 10 erg / (g. K), the temperature gradient is from the expressions (13) and (14):
 (20)
(20)
Note that the calculation by formula (20) and indirectly confirms the validity of the definitions of w and r, made ??by the expressions (11) – (14).
In Fig. 7 shows that at equal pressures, surface temperature and dry transparent troposphere always slightly higher than the moist and absorbing heat. In our example, the average temperature difference reaches +4,7 ° C. This phenomenon seems to be explained by the increased temperature of surface layers of air and drought in the arid desert belt of the Earth, as well as in regions where anticyclones are introduced along with the dry air mass from these arid zones, for example, in the steppes east of the Volga.

Consider the effect of the so-called “greenhouse” gases in the temperature of the troposphere. When replacing the mental nitrogen-oxygen atmosphere on carbon dioxide, but with the same pressure of 1 atm, the average surface temperature is lowered (but not increase, as is commonly believed) by about 2,4 ° C, with lower and temperature throughout the troposphere (Fig. 8). Both curves are based on equations (1) and (5)  1 = 29 g / mol;
1 = 29 g / mol;  2 = 44 g / mol;
2 = 44 g / mol;  1 = 0.1905,
1 = 0.1905,  2 = 0.1423. Similarly, when mental replace the carbon dioxide atmosphere of Venus on the nitrogen-oxygen (at the same pressure 90.9 atm), its surface temperature increases from 735 to 930 K. This shows that the saturation of the atmosphere with carbon dioxide, other things being equal, not always lead to an increase, but only to a reduction of greenhouse effect and the average temperature throughout the thickness of the planet. Explained this phenomenon is simple: the molar mass of carbon dioxide is 1.5 times higher, and its specific heat capacity Cp of about 1.2 times lower than that of terrestrial air. As a result, it follows from equation (9), the adiabatic index for a carbon dioxide atmosphere under otherwise equal conditions is approximately 1.34 times less than for the moist air of nitrogen-oxygen composition. Additional, the increase of the absorption of heat by carbon dioxide leads to an increase of the correction factor Cr and, consequently, to an additional decrease of the adiabatic index
2 = 0.1423. Similarly, when mental replace the carbon dioxide atmosphere of Venus on the nitrogen-oxygen (at the same pressure 90.9 atm), its surface temperature increases from 735 to 930 K. This shows that the saturation of the atmosphere with carbon dioxide, other things being equal, not always lead to an increase, but only to a reduction of greenhouse effect and the average temperature throughout the thickness of the planet. Explained this phenomenon is simple: the molar mass of carbon dioxide is 1.5 times higher, and its specific heat capacity Cp of about 1.2 times lower than that of terrestrial air. As a result, it follows from equation (9), the adiabatic index for a carbon dioxide atmosphere under otherwise equal conditions is approximately 1.34 times less than for the moist air of nitrogen-oxygen composition. Additional, the increase of the absorption of heat by carbon dioxide leads to an increase of the correction factor Cr and, consequently, to an additional decrease of the adiabatic index  (CO 2), and this in turn leads to further reduction in temperature.
(CO 2), and this in turn leads to further reduction in temperature.

A similar, although slightly less cooling should take place in an atmosphere saturated with methane.
Physics phenomena is that the absorption of greenhouse gases infrared radiation heats the air mass, which increases the heat transfer by convection.
Thus, we emphasize again that the saturation of the atmosphere with carbon dioxide or methane could lead only to an acceleration of the convective mass transfer in the troposphere and cooling, but not to an increase in its average temperature and global warming. In addition, for the same total mass of carbon dioxide specific heat of the atmosphere is always smaller than the nitrogen-oxygen. At the same time because of the higher density of carbon dioxide compared with the Earth’s atmosphere is carbon dioxide air thinner and less heat keeps the planet’s surface. It turns out that the conventional wisdom on climate warming in the accumulation of atmospheric CO 2 and other “greenhouse” gases are a myth, realistic is the accumulation of CO 2 under otherwise equal conditions, can lead only to a colder climate.
On the influence of anthropogenic factors
According to various estimates, at the present time due to combustion of fossil fuels into the atmosphere comes about 5-7 billion tons of carbon dioxide, or 1,4-1,9 billion tonnes of pure carbon, which not only reduces the heat capacity of the atmosphere, but also slightly increases its total the pressure. These factors act in opposite directions, resulting in average surface temperature varies very little. For example, if a doubling of CO 2 concentration in the atmosphere from 0.035 to 0.07% (by ??volume), which is expected by 2100, the pressure should be increased to 15 Pa, which will cause a temperature rise of about 7.8. 10 -3 K. If we consider that most of the coming of carbon dioxide, Henry’s law, is dissolved in ocean waters and further hydration of rocks of the oceanic crust is bound in carbonates, it turns out that, together with the carbon in carbonates and becomes part of the atmospheric oxygen. Then, instead of a weak increase in atmospheric pressure can be expected to reduce the minor and, therefore, a weak cooling of climate (but not the significant warming, as environmentalists suggest orthodox). In addition, some carbon dioxide hydration of rocks of the oceanic crust is reduced to methane [17]. In real terms, however, the vital functions of plants should be almost fully offset the impaired person to restore the balance and climatic balance.
From these estimates it follows an important practical conclusion: even large man-made emissions of carbon dioxide into the atmosphere practically do not change the averaged performance of the thermal regime of the Earth and its greenhouse effect. Thus, the prevailing view of a substantial effect of anthropogenic emissions of carbon dioxide on global warming is a myth, really, these emissions do not affect Earth’s climate. Moreover, the increase of carbon dioxide in Earth’s atmosphere is certainly a useful factor increasing agricultural productivity and encouraging more efficient recovery of plant mass in areas of deforestation.
Similar conclusions were reached, and many U.S. scientists who have studied climate change in different regions of North America. Former president of the National Academy of Sciences of the USA prof. F. Seitz has prepared a petition to the U.S. government scientists to renounce international agreements on global warming concluded in Kyoto (Japan) in December 1997, and other similar agreements. In the petition reads in part: “There is no convincing scientific evidence that anthropogenic emissions of carbon dioxide, methane or other greenhouse gases is causing or may in the foreseeable future cause catastrophic heating of the Earth’s atmosphere and disruption of its climate. In addition, there is substantial scientific evidence showing that the increase in atmospheric carbon dioxide leads to a positive impact on the natural growth of plants and animals in the environment of the Earth. “
Copostavlenie averaged surface temperatures in the Northern Hemisphere (middle England) with the magnetic activity of the Sun (according to observations of sunspots) over the period 1750-1970 gg. shows that these temperatures are directly correlated with the graph of solar activity. We should not forget that the observed warming is now a secular began in the early XVII century. When a man-made emissions of carbon dioxide in the atmosphere could not even speak. Moreover, this local warming observed in the general background of many years of cooling. The overall reduction in the temperature of bottom waters of the ocean (Fig. 9 [18]), undoubtedly due to cooling of the Earth’s climate and the emergence of some 40-38 million years ago in the Antarctic glaciers and their first extensive development in the Oligocene and subsequent periods.

In the Pliocene-Quaternary glaciation began and the northern regions, which quickly led to a lowering of bottom water temperature to nearly 0 ° C. The total decrease in temperature over the last 70-60 million years, probably due to the removal of nitrogen from the atmosphere and bind it in soils and sediments azotpogloschayuschimi bacteria and organic matter. At the same time a general cooling of the climate at the present time is not compensated for even a gradual increase in solar luminosity [19]. The same is confirmed by many modern high-precision observations, including satellite (Fig. 10) showing the inverse – a weak cooling of the climate [24]. Records of surface temperature (in the U.S.) show that in 1996 and 1997. As 1938 and 1956., Were cold years of the twentieth century [21].

The influence of ocean carbon dioxide in the atmosphere
In the oceanic waters of dissolved carbon dioxide (in the form of ions HCO 3 -) is almost 59 times greater than it is contained in the atmosphere [12]. It can be shown [17] that the temperature rise of modern ocean by 1 ° C leads to an increase in the partial pressure of atmospheric CO 2 of approximately 13.6. 10 -6 atm (ie, at 13,6 ppm) [ppm – one millionth share. – Ed.], Whereas during the Quaternary glaciations lowered the average ocean temperature to 277 K it was equal to 12,5 ppm. If we compare the average values ??of the partial pressures of CO 2 during glacial and interglacial periods, it appears that their difference can reach about 50-52 ppm. But it is precisely this order of the differential pressure of CO 2 and were found in air bubbles when drilling the Antarctic ice sheet at the station “Vostok” (Fig. 11). It is interesting to note that in the warm Cretaceous period, when the average temperature of ocean water could go up to +17 ° C (291 K), the partial pressure of carbon dioxide was up to 610 ppm, ie, 1.33 times higher than at present.

In Fig. 11. Correlation of changes in concentration of carbon dioxide from the air temperature fluctuations over the last 420 thousand years at the Antarctic station “Vostok” from the core hole in the ice, drilled to a depth of 3623 m (the time is directed from right to left). As can be seen, the trend of the temperature curve (dashed lines) observed a general cooling of the climate over the past 420 thousand years, although the extent of local temperature fluctuations between glacial and interglacial epochs stadialami and reaches 10 ° C. Proceedings of the temperature curve ahead of the changes of CO 2 for about 600 years. The delay curve of CO 2 in cold weather climate and much higher even in the eyes. We currently live in an age decrease in temperature and increase or stabilize CO 2 concentrations in the atmosphere
Considering the problem of the greenhouse effect, impossible to ignore the arguments and ideas of the followers of S. Arrhenius direct action of carbon dioxide on the temperature of the troposphere. Yes, the CO 2 content in air samples from the ancient layers of firn of Greenland and Antarctica show that during interglacial warming concentration of this gas in the atmosphere is always increasing. In a much greater extent, this effect has been observed in warm climatic period, for example, in the Cretaceous. However, it follows from the above data, the proponents of the classical approach is clearly confused cause and effect, for raising or lowering the partial pressure of CO 2 in the atmosphere are not the cause but the consequence of temperature changes. A close examination shows that the curve of temperature fluctuations is clearly faster than the corresponding changes in the concentrations of CO 2: temperature fluctuations are the primary, and changes in carbon dioxide in the atmosphere – a consequence of these oscillations.
This is due to the negative temperature dependence of the solubility of CO 2 in ocean waters and Henry’s law, establishing a dynamic equilibrium between the partial pressure of gas in the atmosphere and its concentration in the hydrosphere. Rising temperatures of ocean water leads to partial degassing and the transition of CO 2 from the ocean to the atmosphere, and, conversely, when cold weather increases the solubility of CO 2 in ocean waters. It is interesting to note that the delay changes in the concentrations of CO 2 compared to the temperature variations in Fig. 11 corresponds approximately to the time of complete mixing of ocean water (about a thousand years).
The true causes of temperature changes the Earth’s climate to be found in other processes and phenomena, such as uneven solar radiation (see Fig. 10), the precession of the Earth’s own rotation, the instability of ocean currents or changes in their circulation due to other causes (eg , the periodic desalination or salinity of surface waters of the Arctic Ocean).
In evolutionary terms the same, starting around mid-Mesozoic (about 150-100 million years ago), there is a gradual cooling of the climate. This is due to several factors, including the removal of nitrogen from the atmosphere and linking it to the nitrate and nitrite soil [19] and a corresponding decrease in mass of the atmosphere, as well as continental drift to higher latitudes. We now live in the interglacial stadiale, but we should expect the arrival of a new phase of glaciation – increased severity.
That’s why Earth’s climate change problem to be solved in a systematic and based on a rigorous physical theory, taking into account the evolution of atmospheric composition, geological environments, involving data on fluctuations in solar luminosity, the precession of the Earth’s rotation and oceanographic data, with obligatory consideration of existing in this complex system of feedbacks and not only explain all the climate and the imaginary dependence of atmospheric concentrations of so-called “greenhouse” gases.
The natural origin of the so-called “ozone holes”
Under the “ozone holes” is usually understood areas of the stratosphere in the polar and temperate latitudes, with reduced approximately 20-30% of the ozone concentration. They occur in the winter and spring at places standing stable anticyclones, such as Antarctica or over Yakutia. This is due to the fact that winter is sharply reduced solar radiation, and in the polar latitudes, and it disappears altogether, and over the anticyclonic areas there is a rise of air masses and their overflowing into the stratosphere as a result of the ozone layer over them as if fading away. In the summer the “holes” are sharply reduced or even disappear.
Panic arose only after the end of the 50s. XX century. were quantitatively measure the ozone in the atmosphere. For the first time “ozone hole” was found in Antarctica. Soon there was mass speculation about man-made influences. In this case, however, remained unclear why the most profound and extensive, “ozone holes” are observed in Antarctica, ie in the Southern Hemisphere, whereas the maximum anthropogenic emissions of CFCs occurs in the North as well, as compared with man-made CFCs better nature coming into the atmosphere in a disproportionately large quantities during volcanic eruptions. However, the main “destroyers” of the ozone layer are not CFCs, and methane and hydrogen. Thus, only the reactions of the type of rock serpentinization of the oceanic crust in their hydration are released, according to [10], 6-10 million tons / year, whereas man-made emissions of CFCs does not exceed 100 tons / year. To this should be added to many millions of tons of methane and hydrogen coming from the soil tectonically active regions and tropical forests, as well as methane emitted swamps of the northern regions of Canada and Eurasia. Total mass entering the atmosphere each year of natural gas reaches many tens or even hundreds of millions of tons!
We can therefore conclude that the role of human impact on the ozone layer in Earth’s stratosphere, in which there are “ozone hole” is negligible – about four orders of magnitude below the influence of environmental factors. Therefore, all variations of ozone concentration in the atmosphere are only natural in nature and unrelated to human activity. As shown by Kapitsa and AA Gavrilov [22], the concentration of ozone in the stratosphere varies with seasonal periodicity, and nothing wrong with that. Moreover, in the process of studies have shown that at the equator and in tropical latitudes the ozone concentration was lower than in the most profound “ozone holes” near-polar regions. And there’s no danger to life at these latitudes is not observed. We can conclude that there is no problem of “ozone holes” in the struggle to which, however, spent enormous funds. Thus, according to some estimates, only to meet the obligations under the Montreal Protocol to the Vienna Convention 1985 on the conservation of the ozone layer, Russia must spend about $ 5 billion a year and a one-time loss from the destruction and replacement of equipment that uses CFCs, is about 10-15 billion dollars! This money can be found and better use.
In conclusion, I want to thank the academics Kondratyev support for the idea, Monin for a discussion of problems and SS Grigoryan for a detailed review of the theory, useful tips and suggestions.
Literature
1. Arrhenius S. On the influence of carbonic acid in the air upon the temperature of the ground. – Phil. Mag., 1896, v. 41, 237-276.
2. Budyko, MI The problem of carbon dioxide. – Leningrad: Gidrometeoizdat, 1997.
3. Global Warming: Greenpeace Report. – Moscow: Moscow State University, 1993.
4. The greenhouse effect, climate change and ecosystems. – Leningrad: Gidrometeoizdat, 1989.
5. Khromov, SP, MA Petrosyants Meteorology and climatology. – Moscow: Moscow State University, 1994.
6. Haken H. Synergetics .- Springer-Verlag, 1980.
7. Haken H. Synergetics. Hierarchy of instabilities in self-organizing systems and devices. – Springer-Verlag, 1985.
8. The atmosphere of the Earth: Physical Encyclopedia, Vol 1. – Moscow: Soviet Encyclopedia, 1988.
9. LD Landau, EM Lifshitz Statistical Physics, Part 1 .- Moscow: Nauka, 1979.
10. Sorokhtin OG Greenhouse Effect: The Myth and Reality. – Journal of Natural Sciences, 2001, v. 1, ? 1, 6-21.
11. Feldbaum, AA Introduction to the theory of nonlinear circuits. – M.: Gosenergoizdat, 1948.
12. Voitkevich G., A. Kokin, Miroshnikov AE, Prokhorov VG Handbook of Geochemistry .- Moscow: Nedra, 1990.
13. Naumov GB, Ryzhenko BN, IL No Comments Handbook of thermodynamic quantities (for geologists) .- Moscow: Atomizdat, 1971.
14. Bachinsky AI Putilov VV, Suvorov NP Handbook on the Physics .- M.: GUPI, 1951.
15. The planet Venus (atmosphere, surface and internal structure). – Moscow: Nauka, 1989.
16. Marov MJ Planets in our solar system. – Moscow: Nauka, 1986.
17. Sorokhtin OG, Lein AY, Balanyuk IE Thermodynamics of oceanic hydrothermal systems and the generation of abiogenic methane. – Oceanology, 2001, v. 41, ? 6,. 898-909.
18. Gladenkov JB Stratigraphy Biosphere: Proceedings of the GIN, no. 551. – Moscow: GEOS, 2004.
19. Sorokhtin OG, SA Ushakov Development of the Earth. – Moscow: Moscow State University, 2002.
20. Christy JR, Spencer RW, Braswell WD – Nature, 1997, v. 389, 342-344.
21. Robinson AB, Baliunas SL, Soon W., Robinson ZW Environmental effects of increased atmospheric carbon dioxide. 1998 [info@oism.org; info@marshall.org].
22. Kapitsa, AP, AA Gavrilov Confirmation of the hypothesis about the natural origin of the Antarctic ozone hole. – Dokl. Academy of Sciences, 1999, v. 366, ? 4,. 543-546.

Oleg G. Sorokhtin – a graduate of the Leningrad Mining Institute in 1951, Dr.,., Academician (RANS), Honored Scientist of Russia, Honorary Polar Explorer. After graduating with honors from the LGI worked Hydroproject – hydrogeological exploration carried out by the “great construction projects of communism” (Kuibyshev HES, Turkmen channel). In 1953 he returned to Moscow and began work at the Institute of Physics of the Earth. Participated in three Antarctic expeditions, carried out a deep drilling and seismic studies, attended the Pole of Cold, the geomagnetic pole, opened the Pole of Inaccessibility. Since 1966 he has been working at the Institute of Oceanology. Shirshov USSR Academy of Sciences (RAS). He has participated in many oceanographic expeditions, fell to the bottom of the ocean, explore the underwater volcanoes, hot springs (black smokers). He has more than 300 scientific publications, including publications in “Reports of the USSR Academy of Sciences / RAS” and “Nature.” He was awarded the Order of Labor Red Banner, medals. He has two sons, two grandsons (the youngest – a year) and a granddaughter. The youngest son – Doctor of sciences, Professor of Murmansk State University (Apatity), corresponding member of Academy of Natural Sciences.
When referring to physical values ??by using units of different systems, as well as Common. Revision retained by the author referred to the unit. Keep in mind that 1 cal  4.2 J, 1 erg = 10 -7 J, 1 atm = 101.32 kPa.
4.2 J, 1 erg = 10 -7 J, 1 atm = 101.32 kPa.
]






Rog, why not just put a link to the Google Translate page where there is no mash-up, at least no problem in just reading?
FWIW, here’s the introduction.
The idea of heating the earth’s atmosphere by greenhouse gases was first expressed in the late XIX century, the famous Swedish scientist Arrhenius S. [1] and since the obvious is taken for granted, with little or no verification [2-5]. This view is now completely dominates the conclusions of the Intergovernmental Panel on Climate Change (IPCC), Greenpeace, the UN Environment Programme (UNEP), World Meteorological Organization (WMO), as well as the withdrawal of Russian environmental and scientific organizations. The same view was fully supported by the decisions of international environmental conventions, in Rio de Janeiro (Brazil) in 1992 and in Kyoto (Japan) in 1997 is projected proponents of these ideas, by 2100, warming could reach 2.5 -5 ° C and cause sea level rise of 0.6 m -1, which already may be a problem for densely populated areas of the continental coasts, as well as for gas and oil production in lowland areas and most of the coasts of northern Russia. Projected, and other harmful consequences for the nature of global warming (the expansion of deserts, the disappearance of permafrost, soil erosion, etc.).
Fears of similar catastrophic events, the pressure of environmental organizations, and often simply speculation on this subject makes governments of developed countries to allocate huge resources to fight the effects of global warming, allegedly linked to anthropogenic emissions to the atmosphere of “greenhouse gases”. And how justified are these costs? Not if we’re fighting “quixotic”?
On closer acquaintance with this problem it turned out that the theory of greenhouse effect as such until the 90s. the last century did not exist, and all calculations of the effect of CO 2 and other greenhouse gases in the Earth’s climate was carried out on different intuitive models with the introduction of numerous and not always stable parameters [4]. In this case the uncertainties in the estimates of various parameters of the model adopted (and they number at least 30) actually make the solution of the problem incorrectly. We decided to use a synergistic approach [6, 7] and an analysis of the most common positions, representing the Earth’s atmosphere as an open dissipative (scattering power) system described by nonlinear equations of mathematical physics.
Good plan Lucy
http://translate.google.com/translate?sl=ru&tl=en&js=n&prev=_t&hl=en&ie=UTF-8&layout=2&eotf=1&u=http%3A%2F%2Ffiz.1september.ru%2Farticlef.php%3FID%3D200501111
Heh, there’s a team of editors on the job now. Tim’s in the middle of doing something to it. I’m going to sit back with my glass of Russian Vodka and relax. 🙂
…and I’m successfully copy-pasting it into OpenOffice. Will email.
ps got any vodka to share? try cutting and pasting that 🙂
[Reply] I’ll attach a picture of the bottle to the return email. Mind you don’t spill any on the keyboard, the letters will come unstuck 🙂
Lucy, sit back and enjoy a glass. Tim wants to do it.
Thanks mate. 🙂
I’ve already emailed it to you in .doc and .odt.
I use Chrome and was given the option of translating from Russian to English, clicked Yes, and now I see a very readable article in English.
[reply] Good stuff, enjoy. 🙂
This is going to get interesting
“Thus, we emphasize again that the saturation of the atmosphere with carbon dioxide or methane could lead only to an acceleration of the convective mass transfer in the troposphere and cooling, but not to an increase in its average temperature and global warming. In addition, for the same total mass of carbon dioxide specific heat of the atmosphere is always smaller than the nitrogen-oxygen. At the same time because of the higher density of carbon dioxide compared with the Earth’s atmosphere is carbon dioxide air thinner and less heat keeps the planet’s surface. It turns out that the conventional wisdom on climate warming in the accumulation of atmospheric CO 2 and other “greenhouse” gases are a myth, realistic is the accumulation of CO 2 under otherwise equal conditions, can lead only to a colder climate.”
Any reason this was published in 2005 but is just hitting the blog sphere? Is there a back story?
ATTENTION: Rog emailed me Lucy’s documents, turned into PDF and is now linked at top of article.
Tell me if this is okay.
Next problem is sort out the article. Can do reasonable direct post *but* this references the Russian server and the sysop there might be unhappy about the load. See what I can do.
There’s a version of this paper in his book, translated into English by Elsevier. You can see most of the pages at GoogleBooks here: http://books.google.com/books?id=FbvNhOUVMBkC&lpg=PP1&pg=PA133#v=onepage&q&f=false
Thanks Tim, downloaded and need to study. Reminds me of some other Russian articles which have not gained traction in English language journals.
There has been an underlaying theme that Russian scientists do not agree with AGW. On my laptop and do not have access to my reference file. Can not remember the website of Dr Anastassia Makarieva who was criticised for an attempt to publish an article about hurricanes formation.
Mike WIlliams says: January 23, 2012 at 11:56 pm
“Any reason this was published in 2005 but is just hitting the blog sphere? Is there a back story?”
Variants have been around for ages and discussed at length, usually under Chilingar’s name. Here’s Eli Rabett dealing with a second iteration in 2008.
Thanks Nick. Rabett’s article is interesting once you get past the preamble:
The Rabett has taken part in a discussion of this paper on the Climate Audit Bulletin Board. C & Co blather a bit about how smart they are, how there are all these wonderful results, mostly from Sorokhin in the the iconic Russian literature, but it all comes back to an adiabatic expansion, and the relationship that can be derived from it between temperature and pressure
(1) T = Cpα
where C is a constant, α = (γ -1)/γ being the ratio between the specific heat at constant pressure and that at constant volume, cp/cv. Since cp-cv = R, the gas constant, 8.314 J/mol-K, α = R/cp. They get all proud of themselves for a trivial rearrangement, taking ratios of T and p at the surface and in the atmosphere to get
(2) T = bα Te ( p/po) α
With Te and po being the temperature and pressure at the surface. At this point, C&Co have reduced everything to determining α and thus cp. They separate cp in to three terms, the specific heat of the gases in the atmosphere and two additional undetermined terms which they associate with radiation and water vapor. They do not separate the sensible and latent heats of water. By comparing the temperature profile of the atmosphere with (2) they obtain a fit to α from which they impute the values of the other two heat capacities by difference. The two additional terms are found to be small compared to the gas heat capacities (essentially 7/2 R). Thus they claim that convection determines the temperature in the atmosphere.
There is lots of silly there. For one, you can’t ignore greenhouse gases absorbing radiation from the ground, and radiating a fair bit of it back. If you do, you find that that solar heating alone cannot keep the surface at a toasty 288 K. C&Co’s only admit to processes which REMOVE energy from the surface, so the surface will cool drastically, way below the case where there is no atmosphere and no convection, because in the chilingarsphere, convection et al, only REMOVE heat from the surface
Eq 2 is the case for what happens in a small system, or out in space, and it is easy to derive from the first law of thermodynamics. But the first law (physicists version where work is done on the surroundings)
(3) dU = δQ – δW
where U is internal energy (all the energy of the atmosphere), Q is heat flowing into and out of the system and W the work done by the system. In the atmosphere we need another term, the potential energy associated with gravity
(4) dU+ mg dz = δQ – δW
where z is the altitude of the packet of air we are looking at m the mass and g the acceleration of gravity. (UPDATE: The term on the left is the total energy of any infinitesmally small packet of air. The first term dU is essentially the kinetic energy of the molecules in the packet, the second their gravitational potential energy. One can, in principal, trade one off for the other. If the total energy stays the same ( δQ – δW=0) if z becomes smaller, that corresponds to the packet being pushed down, then the kinetic energy, and thus the temperature will increase and visa versa.)
Let us warm up (or better said, cool down) with a quick derivation of how gravity enters the picture through the adiabatic lapse rate, the rate at which the air would cool with altitude if there were no water vapor condensation, greenhouse gas radiation and other things (BTW, George C don’t think, those things are very important either).
Atmoz, is a great fan of Met 101. Keeping with first things first, we have to have the equations of hydrostatic equilibrium the derivations are at the link
(5) dp/p =( μ g/RT) dz
where p is the pressure on a packet of air, dp the pressure difference between the top and bottom of the packet, μ the average molar mass of air, R is the gas constant and T the temperature of the packet. In an isothermal atmosphere
(6) p = po exp (-z/zo)
po is the pressure at ground level and zo= (RT/μ g) is called the isothermal scale height (about 8 km for the earth). For an adiabatic expansion temperature changes with altitude as
(7) dT/dz = [(γ -1)/γ] (μ g/R)
For the Earth’s atmosphere, C dT/dz is the dry adiabatic lapse rate, about 8 K/km for the Earth. Eq 6 does a good job of predicting the temperature as a function of altitude for dry regions (the poles, deserts, etc), however, it fails to consider water vapor which heats the atmosphere by contributing the sensible and latent heats, thus the dry.
Chilingar, Khyuk and Sorokhin do not include the effect of the dry adiabatic lapse rate (gravity) in their model. This leads them incorrectly to assigns the entire pressure difference resulting from the dry adiabatic lapse rate to convection. This is incorrect, wrong, mistaken, erroneous, untrue, inaccurate, false, faulty, improper, flawed, inappropriate, unseemly, unbecoming, offensive, indecent, and just plain off.
———————————————
Unfortunately, sincce the re-arrangement of the climate audit server, the link to a discussion with Chillingar et al there gets a 404.
As i mentioned once before the Russians are saying the same thing, the cold bits coming down are colliding with the hot bits going up. This gives you your adiabatic lapse rate and your supposed green house affect, gravity going one way and the puffed up floaties going in the other direction.
The radiation non sense gives you 3/5th’s of 5/8th’s of F All. Gravity strength, the volume of atmosphere and the distance from the sun are the major players. The constitution of the atmosphere is minor and almost negligible in the grand scheme of things.
To the tune of whatever ditty you like.
I’m a little radiation, radiation, radiation, I’m a little radiation, all day long
Down through the mesopause , mesopause , mesopause, down through the , menopause, all day long.
Down through tropopause, tropopause, tropopause, down through,tropopause, all day long.
Now I’m a little kinetic, kinetic, kinetic I’m a little kinetic, all day long
Up through the pressure, pressure, pressure, up through the pressure, all day long.
Back to a little radiation, radiation, radiation, I’m a little radiation, all day long.
—————————————————————————————————————————-
Consider how a refrigerator works – 2 thermostats going down, and a heat pump going up.
What happens to pressured gas through a condenser and then a separation device?
Co2 forcing, what dribble.
Academician Sorokhin shows how the radiatively active gases contribute to the bulk thermodynamic quantities like Cp.
Take a very simple case of the dry adiabatic condition and its lapse rate.
In periods of low or no convection (the neutral atmosphere) the lapse rate is is given by -g/Cp.
The CO2 is still as active as ever but it is naturally fully included in Cp.
I asked Joel Shore how the slab model of mutually radiating slabs would fit into the neutral atmosphere.
Of course, Joel for once, had nothing to say.